question_answer 1) A particle moves in a circle of radius 5 cm with constant speed and time period \[0.2\pi s.\] The acceleration of the particle is
A)
\[25\,m/{{s}^{2}}\]
done
clear
B)
\[36\,m/{{s}^{2}}\]
done
clear
C)
\[5\,m/{{s}^{2}}\]
done
clear
D)
\[15\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 2)
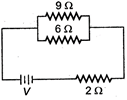
If power dissipated in the \[9\,\Omega \]resistor in the circuit shown in 36W, the potential difference across the \[2\,\Omega \] resistor is
A)
8 V
done
clear
B)
10 V
done
clear
C)
2 V
done
clear
D)
4 V
done
clear
View Answer play_arrow
question_answer 3)
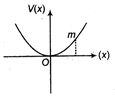
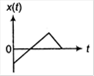
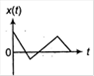
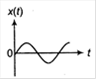
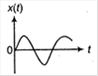
A particle of mass m is released from rest and follows a parabolic path as shown. Assuming that the displacement of the mass from the origin is small, which graph correctly depicts the position of the particle as a function of time?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
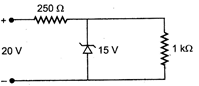
question_answer 4)
A zener diode, having breakdown voltage equal to 15 V, is used in a voltage regulator circuit shown in figure. The current through the diode is
A)
10 mA
done
clear
B)
15 mA
done
clear
C)
20 mA
done
clear
D)
5 mA
done
clear
View Answer play_arrow
question_answer 5) A thin prism of angle \[\text{1}{{\text{5}}^{\text{o}}}\] made of glass of refractive index \[{{\mu }_{1}}=1.5\]is combined with another prism of glass of refractive index \[{{\mu }_{2}}=1.75\]The combination of the prism produces dispersion without deviation. The angle of the second prism should be
A)
\[{{7}^{o}}\]
done
clear
B)
\[{{10}^{o}}\]
done
clear
C)
\[{{12}^{o}}\]
done
clear
D)
\[{{5}^{o}}\]
done
clear
View Answer play_arrow
question_answer 6) A conveyor belt is moving at a constant speed of 2 m/s. A box is gently dropped on it. The coefficient of friction between them is \[\mu =0.5\]The distance that the box will move relative to belt before coming to rest on it taking \[g=10\,m{{s}^{-2}},\]is
A)
1.2m
done
clear
B)
0.6m
done
clear
C)
Zero
done
clear
D)
0.4 m
done
clear
View Answer play_arrow
question_answer 7) An engine pumps water through a hose pipe. Water passes through the pipe and leaves it with a velocity of \[2\,\text{m}{{\text{s}}^{-1}}.\]The mass per unit length of water in the pipe is \[100\,\text{kg}{{\text{m}}^{-1}}.\] What is the power of the engine?
A)
400W
done
clear
B)
200W
done
clear
C)
100 W
done
clear
D)
800 W
done
clear
View Answer play_arrow
question_answer 8) A thin ring of radius R metre has charge q coulomb uniformly spread on it. The ring rotates about its axis with a constant frequency of\[f\]revolution/s. The value of magnetic induction in \[\text{Wb}{{\text{m}}^{-2}}\]at the centre of the ring is
A)
\[\frac{{{\mu }_{0}}qf}{2\pi R}\]
done
clear
B)
\[\frac{{{\mu }_{0}}q}{2\pi fR}\]
done
clear
C)
\[\frac{{{\mu }_{0}}q}{2fR}\]
done
clear
D)
\[\frac{{{\mu }_{0}}qf}{2R}\]
done
clear
View Answer play_arrow
question_answer 9) Which one of the following bonds produces a solid that reflects light in the visible region and whose electrical conductivity decreases with temperature and has high melting point?
A)
Metallic bonding
done
clear
B)
Van der Waals bonding
done
clear
C)
Ionic bonding
done
clear
D)
Covalent bonding
done
clear
View Answer play_arrow
question_answer 10) A particle moves a distance \[x\] in time t according to equation \[x={{(t+5)}^{-1}}.\]The acceleration of particle is proportional to
A)
\[{{(\text{velocity})}^{3/2}}\]
done
clear
B)
\[{{(distance)}^{2}}\]
done
clear
C)
\[{{(distance)}^{-2}}\]
done
clear
D)
\[{{(velocity)}^{2/3}}\]
done
clear
View Answer play_arrow
question_answer 11) A conducting circular loop is placed in a uniform magnetic field, B = 0.025 T with its plane perpendicular to the loop. The radius of the loop is made to shrink at a constant rate of \[\text{1}\,\text{mm}{{\text{s}}^{-1}}.\] The induced emfwhen the radius is 2 cm, is
A)
\[2\pi \mu \,V\]
done
clear
B)
\[\pi \mu \,V\]
done
clear
C)
\[\frac{\pi }{2}\mu V\]
done
clear
D)
\[2\mu V\]
done
clear
View Answer play_arrow
question_answer 12) The activity of a radioactive sample is measured as \[{{N}_{0}}\]counts per minute at \[t=0\]and \[{{N}_{0}}/e\]counts per minute at t = 5 min. The time (in minute)- at which the activity reduces to half its value is
A)
\[{{\log }_{e}}2/5\]
done
clear
B)
\[\frac{5}{{{\log }_{e}}2}\]
done
clear
C)
\[5\,{{\log }_{10}}2\]
done
clear
D)
\[5\,{{\log }_{e}}2\]
done
clear
View Answer play_arrow
question_answer 13) The speed of a projectile at its maximum height is half of its initial speed. The angle of projection is
A)
\[{{60}^{o}}\]
done
clear
B)
\[{{15}^{o}}\]
done
clear
C)
\[{{30}^{o}}\]
done
clear
D)
\[{{45}^{o}}\]
done
clear
View Answer play_arrow
question_answer 14) From a circular disc of radius R and mass 9M, a small disc of mass M and radius \[\frac{R}{3}\]is removed concentrically. The moment of inertia of the remaining disc about an axis perpendicular to the plane of the disc and passing through its centre is
A)
\[\frac{40}{9}M{{R}^{2}}\]
done
clear
B)
\[M{{R}^{2}}\]
done
clear
C)
\[4M{{R}^{2}}\]
done
clear
D)
\[\frac{4}{9}M{{R}^{2}}\]
done
clear
View Answer play_arrow
question_answer 15) A particle moves in \[x-y\]plane according to rule \[x=a\sin \omega t\]and \[y=a\cos \omega t.\]The particle follows
A)
an elliptical path
done
clear
B)
a circular path
done
clear
C)
a parabolic path
done
clear
D)
a straight line path inclined equally to \[x\] and y-axes
done
clear
View Answer play_arrow
question_answer 16) A closely wound solenoid of 2000 turns and area of cross-section \[1.5\times {{10}^{-4}}{{m}^{2}}\]carries a current of 2.0 A. It is suspended through its centre and perpendicular to its length, allowing it to turn in a horizontal plane in a uniform magnetic field \[5\times {{10}^{-2}}\]making an angle of \[{{30}^{o}}\] with the axis of the solenoid. The torque on the solenoid will be
A)
\[3\times {{10}^{-3}}N-m\]
done
clear
B)
\[1.5\times {{10}^{-3}}N-m\]
done
clear
C)
\[1.5\times {{10}^{-2}}N-m\]
done
clear
D)
\[3\times {{10}^{-2}}N-m\]
done
clear
View Answer play_arrow
question_answer 17) The decay constant of a radio isotope is \[\lambda .\] If \[{{A}_{1}}\]and \[{{A}_{2}}\]are its activities at times \[{{t}_{1}}\]and \[{{t}_{2}}\]respectively, the number of nuclei which have decayed during the time \[({{t}_{1}}-{{t}_{2}})\]
A)
\[{{A}_{1}}{{t}_{1}}-{{A}_{2}}{{t}_{2}}\]
done
clear
B)
\[{{A}_{1}}-{{A}_{2}}\]
done
clear
C)
\[\frac{({{A}_{1}}-{{A}_{2}})}{\lambda }\]
done
clear
D)
\[\lambda ({{A}_{1}}-{{A}_{2}})\]
done
clear
View Answer play_arrow
question_answer 18) Susceptibility of ferromagnetic substance is
A)
\[>1\]
done
clear
B)
\[<1\]
done
clear
C)
Zero
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 19) What is the angular velocity of earth?
A)
\[\frac{2\pi }{86400}\text{rad/s}\]
done
clear
B)
\[\frac{2\pi }{3600}\text{rad/s}\]
done
clear
C)
\[\frac{2\pi }{24}rad/s\]
done
clear
D)
\[\frac{2\pi }{6400}\text{rad/s}\]
done
clear
View Answer play_arrow
question_answer 20) What is the refractive index of a prism whose angle \[A={{60}^{o}}\]and angle of minimum deviation \[{{d}_{m}}={{30}^{o}}\]?
A)
\[\sqrt{2}\]
done
clear
B)
\[\frac{1}{\sqrt{2}}\]
done
clear
C)
1
done
clear
D)
\[\frac{1}{\sqrt{3}}\]
done
clear
View Answer play_arrow
question_answer 21) A satellite of mass m is placed at a distance r from the centre of earth (mass M). The mechanical energy of the satellite is
A)
\[-\frac{GMm}{r}\]
done
clear
B)
\[\frac{GMm}{r}\]
done
clear
C)
\[\frac{GMm}{2r}\]
done
clear
D)
\[-\frac{GMm}{2r}\]
done
clear
View Answer play_arrow
question_answer 22) A cell of constant emf first connected to a resistance \[{{R}_{1}}\]and then connected to a resistance \[{{R}_{2}}.\]If power delivered in both cases is same then the internal resistance of the cell is
A)
\[\sqrt{{{R}_{1}}{{R}_{2}}}\]
done
clear
B)
\[\sqrt{\frac{{{R}_{1}}}{{{R}_{2}}}}\]
done
clear
C)
\[\frac{{{R}_{1}}-{{R}_{2}}}{2}\]
done
clear
D)
\[\frac{{{R}_{1}}+{{R}_{2}}}{2}\]
done
clear
View Answer play_arrow
question_answer 23) Energy gap between valence band and conduction band of a semiconductor is
A)
Zero
done
clear
B)
infinite
done
clear
C)
1 eV
done
clear
D)
10 eV
done
clear
View Answer play_arrow
question_answer 24) A glass flask having mass 390 g and an interior volume of \[\text{500 c}{{\text{m}}^{\text{3}}}\]floats on water when it is less than half filled with water. The density of the material of the flask is
A)
0.8 g/cc
done
clear
B)
2.8 g/cc
done
clear
C)
1.8 g/cc
done
clear
D)
0.28 g/cc
done
clear
View Answer play_arrow
question_answer 25) The angle of minimum deviation \[{{\delta }_{m}}\] for an equilateral glass prism is \[\text{3}{{\text{0}}^{\text{o}}}\text{.}\] Refractive index of the prism is
A)
\[1/\sqrt{2}\]
done
clear
B)
\[\sqrt{2}\]
done
clear
C)
\[2\sqrt{2}\]
done
clear
D)
\[1/2\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 26) When the wavelength of sound changes from 1 m to 1.01 m, the number of beats heard are 4. The velocity of sound is
A)
404 m/s
done
clear
B)
4.04 m/s
done
clear
C)
414 m/s
done
clear
D)
400 m/s
done
clear
View Answer play_arrow
question_answer 27) At what height h above earth, the value of g becomes g/2? (R = Radius of earth)
A)
\[3R\]
done
clear
B)
\[\sqrt{2}R\]
done
clear
C)
\[(\sqrt{2}-1)R\]
done
clear
D)
\[\frac{1}{\sqrt{2}}R\]
done
clear
View Answer play_arrow
question_answer 28) A freshly prepared radioactive source of half-life 2h emits radiation of intensity which is 64 times the permissible safe level. Calculate the minimum time after which it would be possible to work safely with this source.
A)
12 h
done
clear
B)
24 h
done
clear
C)
6 h
done
clear
D)
130 h
done
clear
View Answer play_arrow
question_answer 29)
The current in the circuit shown in the figure considering ideal diode is
A)
75N
done
clear
B)
\[80\,N\]
done
clear
C)
\[7.5\,N\]
done
clear
D)
\[30\,N\]
done
clear
View Answer play_arrow
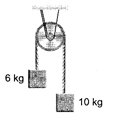
question_answer 30)
The tension in the string in the pulley system shown in the figure is
A)
75N
done
clear
B)
80N
done
clear
C)
7.5 N
done
clear
D)
30 N
done
clear
View Answer play_arrow
question_answer 31) The rest mass of a body is rriQ, if it moves with a velocity of 0.6 c, then its relativistic mass is m, then
A)
\[m<{{m}_{0}}\]
done
clear
B)
\[m={{m}_{0}}\]
done
clear
C)
\[m>{{m}_{0}}\]
done
clear
D)
None of theses
done
clear
View Answer play_arrow
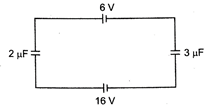
question_answer 32)
What is the potential difference across \[2\mu F\] capacitor in the circuit shown?
A)
12 V
done
clear
B)
4 V
done
clear
C)
6V
done
clear
D)
18 V
done
clear
View Answer play_arrow
question_answer 33) Solar radiation is
A)
transverse electromagnetic wave
done
clear
B)
longitudinal electromagnetic wave
done
clear
C)
stationary wave
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 34) Fundamental frequency of an open pipe is\[{{f}_{0}}\] Fundamental frequency when it is half filled with water is
A)
\[{{f}_{0}}\]
done
clear
B)
\[{{f}_{0}}/2\]
done
clear
C)
\[2{{f}_{0}}\]
done
clear
D)
\[3f\]
done
clear
View Answer play_arrow
question_answer 35) If the rms velocity of a gas is v, then
A)
\[{{v}^{2}}T=\] constant
done
clear
B)
\[{{v}^{2}}/T=\]constant
done
clear
C)
\[v{{T}^{2}}=\]constant
done
clear
D)
v is independent of T
done
clear
View Answer play_arrow
question_answer 36) Which one of the following statement is false?
A)
Pure Si doped with trivalent impurities gives a p-type semiconductor
done
clear
B)
Majority carriers in a n-type semiconductor are holes
done
clear
C)
Minority carriers in a p-type semiconductor are electrons
done
clear
D)
The resistance of intrinsic semiconductor decreases with increase of temperature
done
clear
View Answer play_arrow
question_answer 37) The displacement of a particle along the \[x-\]axis is given by \[x=a{{\sin }^{2}}\omega t.\]The motion of the particle corresponds to
A)
simple harmonic motion of frequency\[\omega /\pi \]
done
clear
B)
simple harmonic motion of frequency\[3\omega /2\pi \]
done
clear
C)
non simple harmonic motion
done
clear
D)
simple harmonic motion of frequency\[\omega /2\pi \]
done
clear
View Answer play_arrow
question_answer 38) The radii of circular orbits of two satellites A and B of the earth are 4R and R, respectively If the speed of satellite A is 3v, then the speed of satellite B will be
A)
\[3v/4\]
done
clear
B)
\[6v\]
done
clear
C)
\[12v\]
done
clear
D)
\[3v/2\]
done
clear
View Answer play_arrow
question_answer 39) A beam of cathode rays is subjected to crossed electric (E) and magnetic fields . The fields are adjusted such that the beam is not deflected. The specific charge of the cathode rays is given by
A)
\[\frac{{{B}^{2}}}{2V{{E}^{2}}}\]
done
clear
B)
\[\frac{2V{{B}^{2}}}{{{E}^{2}}}\]
done
clear
C)
\[\frac{2V{{E}^{2}}}{{{B}^{2}}}\]
done
clear
D)
\[\frac{{{E}^{2}}}{2V{{B}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 40) A ball is dropped from a high rise platform at t = 0 starting from rest. After 6 s another ball is thrown downwards from the same platform with a speed v. The two balls meet at t = 18 s. What is the value of v?(Take \[g=10\,m{{s}^{-2}}\])
A)
\[74\,m{{s}^{-1}}\]
done
clear
B)
\[64\,m{{s}^{-1}}\]
done
clear
C)
\[84\,m{{s}^{-1}}\]
done
clear
D)
\[94\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 41) An alpha nucleus of energy \[\frac{1}{2}m{{v}^{2}}\]bombards a heavy nuclear target of charge Ze. Then the distance of closest approach for the alpha nucleus will be proportional to
A)
\[\frac{1}{Ze}\]
done
clear
B)
\[{{v}^{2}}\]
done
clear
C)
\[\frac{1}{m}\]
done
clear
D)
\[\frac{1}{{{v}^{4}}}\]
done
clear
View Answer play_arrow
question_answer 42) A lens having focal length \[f\] and aperture of diameter d forms an image of intensity \[I.\] Aperture of diameter \[\frac{d}{2}\] in central region of 2i lens is covered by a black paper. Focal length of lens and intensity of image now will be respectively
A)
\[f\]and \[\frac{I}{4}\]
done
clear
B)
\[\frac{3f}{4}\]and \[\frac{I}{2}\]
done
clear
C)
\[f\]and \[\frac{3I}{4}\]
done
clear
D)
\[\frac{f}{2}\]and \[\frac{I}{2}\]
done
clear
View Answer play_arrow
question_answer 43) A galvanometer has a coil of resistance m \[100\,\Omega \] and gives a full scale deflection for 30 mA current. If it is to work as a voltmeter of 30 V range, the resistance required to be added will be
A)
\[900\,\Omega \]
done
clear
B)
\[1800\,\Omega \]
done
clear
C)
\[500\,\Omega \]
done
clear
D)
\[100\,\Omega \]
done
clear
View Answer play_arrow
question_answer 44) A gramophone record is revolving with an angular velocity \[\omega .\] A coin is placed at a distance r from the centre of the record. The static coefficient of friction is \[\mu .\] The coin will revolve with the record if
A)
\[r=\mu g{{\omega }^{2}}\]
done
clear
B)
\[r<\frac{{{\omega }^{2}}}{\mu g}\]
done
clear
C)
\[r\le \frac{\mu g}{{{\omega }^{2}}}\]
done
clear
D)
\[r\ge \frac{\mu g}{{{\omega }^{2}}}\]
done
clear
View Answer play_arrow
question_answer 45) Which of the following statement is false for the properties of electromagnetic waves?
A)
Both electric and magnetic field vectors attain the maxima and minima at the same place and same time
done
clear
B)
The energy in electromagnetic wave is divided equally between electric and magnetic vectors
done
clear
C)
Both electric and magnetic field vectors are parallel to each other and perpendicular to the direction of propagation of wave
done
clear
D)
These waves do not require any material medium for propagation
done
clear
View Answer play_arrow
question_answer 46) Assertion Magnetic field interacts with a moving charge and not with a stationary charge. Reason A moving charge produces a magnetic field.
A)
If the Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 47) Assertion There is no current in the metals in the absence of electric field. Reason Motion of free electrons are randomly.
A)
If the Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 48) Assertion Particle velocity and wave velocity both are independent of time. Reason For the propagation of wave motion, the medium must have the properties of elasticity and inertia.
A)
If the Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 49) Assertion For higher temperature, the peak emission wavelength of a black body shifts to lower wavelength. Reason Peak emission wavelength of a black body is proportional to the fourth power of temperature.
A)
If the Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 50) Assertion Displacement of a body may be zero when distance travelled by it is not zero. Reason The displacement is the longer distance between initial and final position.
A)
If the Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 51) Diborane reacts with ammonia under different conditions to give a variety of products. Which one amo ng the following is not formed in these reactions?
A)
\[{{B}_{2}}{{H}_{6}}.2N{{H}_{3}}\]
done
clear
B)
\[{{B}_{12}}{{H}_{12}}\]
done
clear
C)
\[{{B}_{3}}{{N}_{3}}{{H}_{6}}\]
done
clear
D)
\[{{(BN)}_{n}}\]
done
clear
View Answer play_arrow
question_answer 52) Helium mixed with oxygen is used in the treatment of
A)
beri beri
done
clear
B)
burning feet
done
clear
C)
joints burning
done
clear
D)
asthma
done
clear
View Answer play_arrow
question_answer 53) Which one of the following of 2, 3-butane idol is enantiomeric?
A)
2R, 3R and 2S, 3S
done
clear
B)
2S, 3S and 25, 3R
done
clear
C)
2R, 3R and 2R, 35
done
clear
D)
2S, 3S and 2R, 35
done
clear
View Answer play_arrow
question_answer 54) The AT/GC ratio in human beings is (where A = adenine, T = thymine, G = Guanine, C = cytosine)
A)
1
done
clear
B)
1.52
done
clear
C)
9.3
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 55) If \[BaC{{l}_{2}}\]ionizes to an extent of 80% in aqueous solution, the value of vant Hoff factor is
A)
2.6
done
clear
B)
0.24
done
clear
C)
0.8
done
clear
D)
2.4
done
clear
View Answer play_arrow
question_answer 56) The \[s{{p}^{3}}\]hybridisation is present on the central atom in
A)
HCHO
done
clear
B)
\[BC{{l}_{3}}\]
done
clear
C)
\[PC{{l}_{3}}\]
done
clear
D)
\[S{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 57) The number of unit cells in the 5.85g crystals of NaCI are
A)
\[1.5\times {{10}^{23}}\]
done
clear
B)
\[1.5\times {{10}^{22}}\]
done
clear
C)
\[3.0\times {{10}^{22}}\]
done
clear
D)
\[3.0\times {{10}^{23}}\]
done
clear
View Answer play_arrow
question_answer 58) The oxidation number of sulphur is -1 in
A)
\[{{H}_{2}}S\]
done
clear
B)
\[Fe{{S}_{2}}\]
done
clear
C)
\[C{{S}_{2}}\]
done
clear
D)
\[C{{u}_{2}}S\]
done
clear
View Answer play_arrow
question_answer 59) For which of the following metals, the electronic configuration of valence shell is not\[{{d}^{6}}\]?
A)
Fe (II)
done
clear
B)
Mn (II)
done
clear
C)
Co (III)
done
clear
D)
Ni (IV)
done
clear
View Answer play_arrow
question_answer 60) 68 g sugar \[({{C}_{12}}{{H}_{22}}{{O}_{11}})\] is dissolved in 1 kg of water. What is the mole fraction of sugar?
A)
0.018
done
clear
B)
0.036
done
clear
C)
0.0018
done
clear
D)
0.0036
done
clear
View Answer play_arrow
question_answer 61) The pH of 0.1 M aqueous ammonia\[({{K}_{b}}=1.8\times {{10}^{-5}})\]is
A)
9.13
done
clear
B)
10.13
done
clear
C)
11.13
done
clear
D)
12.13
done
clear
View Answer play_arrow
question_answer 62) From the following mixtures, which is not a buffer (concentration level 0.5 M)?
A)
\[C{{H}_{3}}COOH+NaOH(2:1)\]
done
clear
B)
\[HCl+N{{H}_{3}}(aq)(1:2)\]
done
clear
C)
\[C{{H}_{3}}COOH+NaOH(1:2)\]
done
clear
D)
\[HCl+N{{H}_{3}}(2:3)\]
done
clear
View Answer play_arrow
question_answer 63) How much volume (in litre) of 3 M NaOH is obtained from 80 g NaOH? (Atomic mass of Na = 23 u)
A)
2.67
done
clear
B)
1.34
done
clear
C)
0.67
done
clear
D)
0.33
done
clear
View Answer play_arrow
question_answer 64) The initial concentration of sugar solution is 0.12M. On doing fermentation the concentration of sugar decreases to 0.06 M in 10 h and to 0.045 M in 15 h. The order of the reaction is
A)
0.5
done
clear
B)
1.0
done
clear
C)
1.5
done
clear
D)
2.0
done
clear
View Answer play_arrow
question_answer 65) For the decomposition of \[{{N}_{2}}{{O}_{5}}\]into \[{{N}_{2}}O\]and \[{{O}_{2}}\] in the presence of Ar, the velocity constant, k is \[k=5\times {{10}^{11}}{{e}^{-30,000/T}}\]For this, the activation energy is (in \[\text{kJ}\,\text{mo}{{\text{l}}^{-1}}\])
A)
2.494
done
clear
B)
24.94
done
clear
C)
249.42
done
clear
D)
2494
done
clear
View Answer play_arrow
question_answer 66) The following equilibrium establishes on heating 0.2 mole of H2 and 1.0 mole of sulphur in 1 L vessel at \[90{{\,}^{o}}C.\] \[{{H}_{2}}(g)+S(s)\rightleftharpoons {{H}_{2}}S(g);K=6.8\times {{10}^{-2}}\] The partial pressure of \[{{H}_{2}}S\]in equilibrium state is
A)
4.20
done
clear
B)
0.42
done
clear
C)
0.21
done
clear
D)
0.042
done
clear
View Answer play_arrow
question_answer 67) An aqueous solution boils at \[100.2{{\,}^{o}}C.\]which temperature this will freeze? \[({{K}_{b}}=0.5{{\,}^{o}}C/m,{{K}_{f}}=1.9{{\,}^{o}}C/m)\]
A)
\[+\,0.76\]
done
clear
B)
\[-\,0.76\]
done
clear
C)
\[-\,0.38\]
done
clear
D)
\[+\,0.38\]
done
clear
View Answer play_arrow
question_answer 68) The lowest \[p{{K}_{a}}\] value is for
A)
phenol
done
clear
B)
m-cresol
done
clear
C)
o-cresol
done
clear
D)
p-cresol
done
clear
View Answer play_arrow
question_answer 69) At room temperature, the least stable compound is
A)
\[C{{H}_{3}}COCl\]
done
clear
B)
\[HCOCl\]
done
clear
C)
\[C{{H}_{3}}COOH\]
done
clear
D)
\[{{(C{{H}_{3}}CO)}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 70) For the conversion of \[C{{H}_{2}}=C{{H}_{2}}\]into \[HOOC.C{{H}_{2}}C{{H}_{2}}COOH,\] the minimum number of steps required are
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 71) Which of the following compounds does not give two isomer compounds on reaction with \[N{{H}_{2}}OH?\]
A)
\[C{{H}_{3}}CO{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
\[PhCOC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 72) From the following which is not a reactant, reagent or product in Hofmann reaction?
A)
\[RCON{{H}_{2}}\]
done
clear
B)
\[RN{{H}_{2}}\]
done
clear
C)
\[B{{r}_{2}},OH\]
done
clear
D)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 73) The total number of isomers for cyclic alcohol \[{{C}_{4}}{{H}_{7}}OH\]is
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 74) The least stable free radical is
A)
\[\overset{\centerdot }{\mathop{C}}\,{{H}_{2}}C{{H}_{2}}CH{{\left( C{{H}_{3}} \right)}_{2}}\]
done
clear
B)
\[C{{H}_{3}}\underset{\centerdot }{\mathop{C}}\,HCH{{\left( C{{H}_{3}} \right)}_{2}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}\underset{\centerdot }{\mathop{C}}\,{{(C{{H}_{3}})}_{2}}\]
done
clear
D)
\[\overset{\centerdot }{\mathop{C}}\,{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 75) The suitable reagent for the reduction of \[{{C}_{2}}{{H}_{5}}COOH\]into \[{{C}_{3}}{{H}_{7}}OH\]is
A)
\[B{{H}_{3}}/THF\] and\[{{H}_{3}}{{O}^{+}}\]
done
clear
B)
\[NaB{{H}_{4}}\]
done
clear
C)
\[Na/EtOH\]
done
clear
D)
\[{{H}_{2}}/catalyst\]
done
clear
View Answer play_arrow
question_answer 76) The blue colour of acidic solution of \[C{{r}_{2}}O_{7}^{2-}\]is not changed into green by
A)
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}OH\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}CHOH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
D)
\[{{(C{{H}_{3}})}_{3}}COH\]
done
clear
View Answer play_arrow
question_answer 77) The reaction of \[{{C}_{2}}{{H}_{5}}Cl\]with Li and \[\text{CuI}\]gives mainly
A)
2-butene
done
clear
B)
1, 3-butadiene
done
clear
C)
n-butane
done
clear
D)
n-butyl chloride
done
clear
View Answer play_arrow
question_answer 78) The reagent which does not convert n-butyl chloride into n-butane is
A)
\[Zn,HCl\]
done
clear
B)
\[LiAl{{H}_{4}}\]
done
clear
C)
\[Mg,\] anhydrous ether,\[{{H}_{2}}O\]
done
clear
D)
\[{{B}_{2}}{{H}_{6}}\] in THF
done
clear
View Answer play_arrow
question_answer 79) The correct order of stability is
A)
pentane < iso -pentane < neo-pentane
done
clear
B)
iso -pentane < neo-pentane < pentane
done
clear
C)
neo-pentane < iso-pentane < pentane
done
clear
D)
pentane < neo-pentane < 150-pentane
done
clear
View Answer play_arrow
question_answer 80) The correct order of boiling point of ethyl dimethyl amine , n-butyl amine and diethyl amineis
A)
\[B>C>A\]
done
clear
B)
\[B>A>C\]
done
clear
C)
\[A>B>C\]
done
clear
D)
\[C>B>A\]
done
clear
View Answer play_arrow
question_answer 81) Which of the following compounds does not give lodoform test?
A)
\[C{{H}_{3}}COC{{H}_{2}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[~PhC{{H}_{2}}COC{{H}_{3}}\]
done
clear
C)
\[M{{e}_{3}}CCOC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 82) The species which acts as both nucleophile and electrophile is
A)
\[C{{H}_{3}}CN\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[P{{(C{{H}_{3}})}_{2}}\]
done
clear
D)
\[{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 83) The most basic from the following is
A)
\[N{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
C)
\[N{{F}_{3}}\]
done
clear
D)
\[N{{(Si{{H}_{3}})}_{3}}\]
done
clear
View Answer play_arrow
question_answer 84) The main product of the reaction of benzene with lithium in liquid ammonia and \[EtOH\]is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 85) The rate of free radical chlorination of \[C{{H}_{4}}\] is
A)
equal to\[C{{D}_{4}}\]
done
clear
B)
double the rate of \[C{{D}_{4}}\]
done
clear
C)
12 times the rate of \[C{{D}_{4}}\]
done
clear
D)
less than the rate of\[C{{D}_{4}}\]
done
clear
View Answer play_arrow
question_answer 86) The first ionisation energy difference maximum for which pair?
A)
\[Na,Mg\]
done
clear
B)
\[K,Ca\]
done
clear
C)
\[Rb,Sr\]
done
clear
D)
\[Cs,Ba\]
done
clear
View Answer play_arrow
question_answer 87) The ratio of third Bohr orbit radii second Bohr orbit radius for the hydrogen atom is
A)
0.5
done
clear
B)
1.5
done
clear
C)
0.75
done
clear
D)
2.25
done
clear
View Answer play_arrow
question_answer 88) The principal quantum number of \[Mn\] those valence shell orbits in which electrons are filled
A)
4, 3
done
clear
B)
4, 4
done
clear
C)
3, 3
done
clear
D)
5, 4
done
clear
View Answer play_arrow
question_answer 89) From the generally known oxidation states, the oxidation number is maximum for
A)
\[Mn\]
done
clear
B)
\[Cu\]
done
clear
C)
\[Sn\]
done
clear
D)
\[Sc\]
done
clear
View Answer play_arrow
question_answer 90) The number of molecules in 180 g of heavy water are
A)
\[6.02\times {{10}^{24}}\]
done
clear
B)
\[6.02\times {{10}^{22}}\]
done
clear
C)
\[5.42\times {{10}^{24}}\]
done
clear
D)
\[5.42\times {{10}^{23}}\]
done
clear
View Answer play_arrow
question_answer 91) Which of the following is reduced by\[{{H}_{2}}{{O}_{2}}\]?
A)
\[C{{l}_{2}}\]
done
clear
B)
\[{{[Fe{{(CN)}_{6}}]}^{4-}}\]
done
clear
C)
\[N{{H}_{2}}OH\]
done
clear
D)
\[SO_{3}^{2-}\]
done
clear
View Answer play_arrow
question_answer 92) The maximum energy molecular orbit filled by electron in nitrogen molecule is/are
A)
\[\sigma 2{{p}_{z}}\]
done
clear
B)
\[\pi 2{{p}_{x}}\approx 2{{p}_{y}}\]
done
clear
C)
\[\overset{*}{\mathop{\pi }}\,2{{p}_{x}}\approx \overset{*}{\mathop{\pi }}\,2{{p}_{y}}\]
done
clear
D)
\[\overset{*}{\mathop{\sigma }}\,2{{p}_{z}}\]
done
clear
View Answer play_arrow
question_answer 93) Which of the following is correct order for density?
A)
\[Cs>Rb>K>Na\]
done
clear
B)
\[Cs>Rb>Na>K\]
done
clear
C)
\[Rb>Cs>K>Na\]
done
clear
D)
\[Rb>Cs>Na>K\]
done
clear
View Answer play_arrow
question_answer 94) The correct order of dipole moment is
A)
\[B{{F}_{3}}<{{H}_{2}}S<{{H}_{2}}O\]
done
clear
B)
\[{{H}_{2}}S<B{{F}_{3}}<{{H}_{2}}O\]
done
clear
C)
\[{{H}_{2}}O<{{H}_{2}}S<B{{F}_{3}}\]
done
clear
D)
\[{{H}_{2}}O<B{{F}_{3}}<{{H}_{2}}S\]
done
clear
View Answer play_arrow
question_answer 95) Which of the following bond has minimum bond energy?
A)
C-H
done
clear
B)
N-H
done
clear
C)
O-H
done
clear
D)
F-H
done
clear
View Answer play_arrow
question_answer 96) Assertion\[N_{3}^{-}\]is a weaker base than \[NH_{2}^{-}.\] Reason The lone pair of electrons on N atom in \[N_{3}^{-}\]is in a\[s{{p}^{2}}-\]orbital while in \[NH_{2}^{-}\]it is in an \[s{{p}^{3}}-\]orbital.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 97) Assertion Chlorine when solidifies does not have zero entropy even at absolute zero. Reason Chlorine is a pungent smelling gas and it is difficult to solidify it.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 98) Assertion In the E2 elimination, \[\beta -H\]and leaving group should be antiperiplanar. Reason In the E2 elimination, base always abstracts hindered \[\beta -H.\]
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 99) Assertion In chemisorptidn, adsorption first increase and then decreases with temperature. Reason Heat keeps on providing more and more activation energy.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 100) Assertion p-dichlorobenzene is less soluble in organic solvents than the corresponding o-isomer. Reason o-dichlorobenzene is polar while p-dichlorobenzene is not.
A)
If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 101)
Consider the following statements concerning food chains. I. Removal of 80% tigers from an area resulted in greatly increased growth of vegetation. II. Removal of most of the carnivores resulted in an increased population of deers. III. The length of food chains is generally limited to 3-4 trophic levels due to energy loss. IV. The length of food chains may vary from 2-8 trophic levels.
Which of the above statements are correct?
A)
II and IV
done
clear
B)
I, III and V
done
clear
C)
II and IV
done
clear
D)
I and V
done
clear
View Answer play_arrow
question_answer 102)
Given below are four methods (A-D) and their modes of action (1-4) in-achieving contraception. Select their correct matching from the four options that follow Method Mode of action A. The pill 1. Prevents sperms reaching cervix B. Condom 2. Prevents implantation C. Vasectomy 3. Prevents ovulation D. Copper-T 4. Semen contains no sperms
A)
A-3 B-1 C-4 D-2
done
clear
B)
A-4 B-1 C-2 D-3
done
clear
C)
A-3 B-4 C-1 D-2
done
clear
D)
A-2 B-3 C-1 D-4
done
clear
View Answer play_arrow
question_answer 103)
Carbohydrates are commonly found as starch in plant storage organs. Which of the following five properties of starch (I-V) make it useful as a storage material? I Easily translocated II Chemically non-reactive III Easily digested by animals IV Osmotically inactive V Synthesized during photosynthesis
The useful properties are
A)
II and III
done
clear
B)
II and IV
done
clear
C)
I, II and V
done
clear
D)
I and V
done
clear
View Answer play_arrow
question_answer 104)
Match the disease in Column I with the appropriate items (pathogen/prevention/ treatment) in Column II Column I Column II A. B. Amoebiasis Diphtheria 1. Treponema pallidum 2. Use only sterilized food and water C. D. Cholera Syphilis 3. DPT vaccine 4. Use oral rehydration therapy
A)
A-1 B-2 C-3 D-4
done
clear
B)
A-2 B-4 C-1 D-3
done
clear
C)
A-2 B-1 C-3 D-4
done
clear
D)
A-2 B-3 C-4 D-1
done
clear
View Answer play_arrow
question_answer 105) Which one of the following is the correct statement regarding the particular psychotropic drug specified?
A)
Hashish causes alter thought perceptions and hallucinations
done
clear
B)
Opium stimulates nervous system and causes hallucinations
done
clear
C)
Morphine leads to delusions and disturbed emotions
done
clear
D)
Barbiturates cause relaxation and temporary euphoria
done
clear
View Answer play_arrow
question_answer 106) Which one of the following is the true description about an animal concerned?
A)
Earthworm- The alimentary canal consists of a sequence of pharynx, oesophagus, tomach, gizzard and intestine
done
clear
B)
Frog- Body divisible into three regions-head, neck and trunk
done
clear
C)
Rat- Left kidney is slightly higher in position than the right one
done
clear
D)
Cockroach- 10 pairs of spiracles (2 pairs on thorax and 8 pairs on abdomen)
done
clear
View Answer play_arrow
question_answer 107) In cockroach, larval and nymphal characters are maintained by
A)
ecdysone
done
clear
B)
salivary glands
done
clear
C)
parotid gland
done
clear
D)
juvenile hormone
done
clear
View Answer play_arrow
question_answer 108) Which of the following is a transparent tissue?
A)
Tendon
done
clear
B)
Fibrous cartilage
done
clear
C)
Hyaline cartilage
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 109) Rh factor is present in
A)
all vertebrates
done
clear
B)
all mammals
done
clear
C)
all reptiles
done
clear
D)
man and rhesus monkey only
done
clear
View Answer play_arrow
question_answer 110) In rabbit, end of a long bone is connected in another by
A)
tendon
done
clear
B)
ligaments
done
clear
C)
muscle
done
clear
D)
cartilage
done
clear
View Answer play_arrow
question_answer 111) Which of the following cell type .is capable of giving rise to other cell types in sponges?
A)
Thesocytes
done
clear
B)
Pinacocytes
done
clear
C)
Cnidocytes
done
clear
D)
Archaeocytes
done
clear
View Answer play_arrow
question_answer 112) Thigmotaxis is not shown by
A)
Paramecium
done
clear
B)
Amoeba
done
clear
C)
Ascaris
done
clear
D)
Hydra
done
clear
View Answer play_arrow
question_answer 113) Which is correctly matched?
A)
Apiculture - Honey bee
done
clear
B)
Pisciculture - Silk moth
done
clear
C)
Sericulture - Fish
done
clear
D)
Aquaculture - Mosquito
done
clear
View Answer play_arrow
question_answer 114) Changes that allow the conversion of larva into adult, is called
A)
metagenesis
done
clear
B)
alternation
done
clear
C)
metamorphosis
done
clear
D)
metastasis
done
clear
View Answer play_arrow
question_answer 115) Animals having a built in thermostat to maintain constant body temperature are known as
A)
biothermic
done
clear
B)
poikilothermic
done
clear
C)
oligothermic
done
clear
D)
homeothermic
done
clear
View Answer play_arrow
question_answer 116) The intermediate host of Schistosoma is
A)
snail
done
clear
B)
mosquito
done
clear
C)
housefly
done
clear
D)
sheep
done
clear
View Answer play_arrow
question_answer 117) The islets of Langerhans are found in
A)
pancreas
done
clear
B)
stomach
done
clear
C)
liver
done
clear
D)
alimentary canal
done
clear
View Answer play_arrow
question_answer 118) The vitamin, which is essential for blood clotting is
A)
vitamin-A
done
clear
B)
vitamin-B
done
clear
C)
vitamin-C
done
clear
D)
vitamin-K
done
clear
View Answer play_arrow
question_answer 119) The female genital pore of Pheretima posthuma located upon the segment
A)
14th
done
clear
B)
16th
done
clear
C)
18th
done
clear
D)
15th
done
clear
View Answer play_arrow
question_answer 120) Polyp phase is absent in
A)
Hydra
done
clear
B)
Aurelia
done
clear
C)
Physalia
done
clear
D)
Obelia
done
clear
View Answer play_arrow
question_answer 121) In frog heart, there are cardiac muscles, which consists of fibres called
A)
Purkinje fibres
done
clear
B)
myonemes
done
clear
C)
telodendria
done
clear
D)
columnae carnae
done
clear
View Answer play_arrow
question_answer 122) Malpighian tubules are
A)
excretory organs of insects
done
clear
B)
excretory organs of frog
done
clear
C)
respiratory organs of insects
done
clear
D)
endocrine glands of insects
done
clear
View Answer play_arrow
question_answer 123) LH and FSH are collectively called
A)
oxytocin
done
clear
B)
somatotropins
done
clear
C)
luteotropic
done
clear
D)
gonadotropins
done
clear
View Answer play_arrow
question_answer 124) Who is known as father of endocrinology?
A)
RH Whittaker
done
clear
B)
Pasteur
done
clear
C)
Einthoven
done
clear
D)
Thomas Addison
done
clear
View Answer play_arrow
question_answer 125) Which of the following provides most evident proof of evolution?
A)
Fossils
done
clear
B)
Morphology
done
clear
C)
Embryo
done
clear
D)
Vestigial organs
done
clear
View Answer play_arrow
question_answer 126) In Mollusca, eye is present over a static called
A)
ostracum
done
clear
B)
operculum
done
clear
C)
ommatophores
done
clear
D)
osphradium
done
clear
View Answer play_arrow
question_answer 127) Among the following, colonial insects are
A)
locusts
done
clear
B)
mosquitoes
done
clear
C)
white ants
done
clear
D)
bed bug
done
clear
View Answer play_arrow
question_answer 128) In Ascaris, the coelom is
A)
schizocoelom
done
clear
B)
pseudocoelom
done
clear
C)
true coelom
done
clear
D)
haemocoelom
done
clear
View Answer play_arrow
question_answer 129) Turbellarians are free living
A)
nematodes
done
clear
B)
cestodes
done
clear
C)
flatworms
done
clear
D)
trematodes
done
clear
View Answer play_arrow
question_answer 130) The characteristic larva of phylum- Coelenterata is
A)
planula
done
clear
B)
cysticercus
done
clear
C)
rhabdiform
done
clear
D)
wriggler
done
clear
View Answer play_arrow
question_answer 131) In rabbit, head of epididymis present at the head of the testis is called
A)
vas defereris
done
clear
B)
cauda epididymis
done
clear
C)
gubernaculum
done
clear
D)
caput epididymis
done
clear
View Answer play_arrow
question_answer 132) Tendons and ligaments are specialized types of
A)
nervous tissue
done
clear
B)
muscular tissue
done
clear
C)
epithelial tissue
done
clear
D)
connective tissue
done
clear
View Answer play_arrow
question_answer 133) In blood, \[C{{O}_{2}}\]in transported majorly as
A)
sodium carbonate
done
clear
B)
carboxyhaemoglobin
done
clear
C)
bicarbonate
done
clear
D)
\[C{{O}_{2}}\]as such
done
clear
View Answer play_arrow
question_answer 134) Animals undergoes inactive stage during winter, is known as
A)
aestivation
done
clear
B)
hibernation
done
clear
C)
adaptation
done
clear
D)
acclimatization
done
clear
View Answer play_arrow
question_answer 135) Kupffer cells are present in
A)
liver
done
clear
B)
small intestine
done
clear
C)
pancreas
done
clear
D)
thyroid gland
done
clear
View Answer play_arrow
question_answer 136) The embryo at 16 celled stage is known as
A)
morula
done
clear
B)
gastrula
done
clear
C)
blastula
done
clear
D)
blastomere
done
clear
View Answer play_arrow
question_answer 137) Contractile vacuole in protozoan Amoeba is meant for
A)
respiration
done
clear
B)
excretion
done
clear
C)
locomotion
done
clear
D)
osmoregulation
done
clear
View Answer play_arrow
question_answer 138) Jumping genes in maize were discovered by
A)
Hugo de Vries
done
clear
B)
Barbara Me Clintock
done
clear
C)
TH Morgan
done
clear
D)
Mendel
done
clear
View Answer play_arrow
question_answer 139) Streptomycin is obtained from
A)
Streptomyces griseus
done
clear
B)
S. aureofaciens
done
clear
C)
S. venezuelae
done
clear
D)
S. ramosus
done
clear
View Answer play_arrow
question_answer 140) Binomial system of nomenclature was given by
A)
Julian Huxley
done
clear
B)
Bentham and Hooker
done
clear
C)
Linnaeus
done
clear
D)
Casper Bauhin
done
clear
View Answer play_arrow
question_answer 141) Indusium is found in
A)
algae
done
clear
B)
ferns
done
clear
C)
moss
done
clear
D)
Cycas
done
clear
View Answer play_arrow
question_answer 142) The vacuole is lined by a membrane called
A)
tonoplast
done
clear
B)
jacket
done
clear
C)
cell membrane
done
clear
D)
tonoplasm
done
clear
View Answer play_arrow
question_answer 143) Agar-agar is obtained from
A)
Chlorella
done
clear
B)
Spirogyra
done
clear
C)
Ulothrix
done
clear
D)
Gelidium
done
clear
View Answer play_arrow
question_answer 144) DNA element with ability to change position is called
A)
cistron
done
clear
B)
transposon
done
clear
C)
intron
done
clear
D)
recon
done
clear
View Answer play_arrow
question_answer 145) Initiation codon is
A)
UUU
done
clear
B)
UGA
done
clear
C)
AUG
done
clear
D)
UAG
done
clear
View Answer play_arrow
question_answer 146) DNA multiplication is called
A)
translation
done
clear
B)
replication
done
clear
C)
transduction
done
clear
D)
transcription
done
clear
View Answer play_arrow
question_answer 147) Duramen is present in
A)
inner region of secondary wood
done
clear
B)
part of sap wood
done
clear
C)
outer region of secondary wood
done
clear
D)
region of pericycle
done
clear
View Answer play_arrow
question_answer 148) In plants, water supply is due to
A)
osmosis
done
clear
B)
imbibition
done
clear
C)
guttation
done
clear
D)
adhesion force
done
clear
View Answer play_arrow
question_answer 149) Programmed cell death is scientifically termed as
A)
autotomy
done
clear
B)
cell lysis
done
clear
C)
apoptosis
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 150) Paraffin wax is
A)
ester
done
clear
B)
acid
done
clear
C)
monohydric alcohol
done
clear
D)
cholesterol
done
clear
View Answer play_arrow
question_answer 151) Which is always present in photochemical smog?
A)
\[{{O}_{3}}\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
\[C{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 152) In cell cycle, during which phase, chromosomes are arranged in equatorial plate?
A)
Metaphase
done
clear
B)
Anaphase
done
clear
C)
Telophase
done
clear
D)
Prophase
done
clear
View Answer play_arrow
question_answer 153) The soil which is transported by wind is known as
A)
colluvial
done
clear
B)
eolian
done
clear
C)
aluvial
done
clear
D)
galcial soil
done
clear
View Answer play_arrow
question_answer 154) Spindle fibre is made up of
A)
tubulin
done
clear
B)
humulin
done
clear
C)
intermediate filament
done
clear
D)
flagellin
done
clear
View Answer play_arrow
question_answer 155) Lichen is the pioneer vegetation on which succession?
A)
Hydrosere
done
clear
B)
Lithosere
done
clear
C)
Psammosere
done
clear
D)
Xerosere
done
clear
View Answer play_arrow
question_answer 156) Law of limiting factors was given by
A)
Leibig
done
clear
B)
Blackman
done
clear
C)
Calvin
done
clear
D)
Arnon
done
clear
View Answer play_arrow
question_answer 157) In Pinus, male cone bears a large number of
A)
ligules
done
clear
B)
anthers
done
clear
C)
microsporophylls
done
clear
D)
megasporophylls
done
clear
View Answer play_arrow
question_answer 158) Induction of flowering by low temnerature treatment is
A)
vernalization
done
clear
B)
cryobiology
done
clear
C)
photoperiodism
done
clear
D)
prumiing
done
clear
View Answer play_arrow
question_answer 159) Decomposers are
A)
autotrophs
done
clear
B)
autoheterotrophs
done
clear
C)
organotrophs
done
clear
D)
heterotrophs
done
clear
View Answer play_arrow
question_answer 160) Cleavage polyembryony occurs in
A)
Pinus
done
clear
B)
Mini Cycas
done
clear
C)
Cycas
done
clear
D)
Ephedra
done
clear
View Answer play_arrow
question_answer 161) Edible part of mushroom is
A)
basidiocarp
done
clear
B)
secondary mycelium
done
clear
C)
primary mycelium
done
clear
D)
tertiary mycelium
done
clear
View Answer play_arrow
question_answer 162) Which of the following plant product is the hardest?
A)
Lignin
done
clear
B)
Cutin
done
clear
C)
Suberin
done
clear
D)
Sporopollenin
done
clear
View Answer play_arrow
question_answer 163) Calyptra is derived from
A)
archegonia
done
clear
B)
capsule
done
clear
C)
antheridia
done
clear
D)
columella
done
clear
View Answer play_arrow
question_answer 164) Clamp connections are observed in
A)
Basidiomycetes
done
clear
B)
Zygomycetes
done
clear
C)
Ascomycetes
done
clear
D)
Oomycetes
done
clear
View Answer play_arrow
question_answer 165) Leaf abscission is caused by
A)
ABA
done
clear
B)
cytokinin
done
clear
C)
auxin
done
clear
D)
gibberellin
done
clear
View Answer play_arrow
question_answer 166) What is the main cause for the extinction of some species in tropical forest?
A)
Deforestation
done
clear
B)
Afforestation
done
clear
C)
Pollution
done
clear
D)
Soil erosin
done
clear
View Answer play_arrow
question_answer 167) Most accepted theory for ascent of sap is
A)
capillarity theory
done
clear
B)
root pressure theory
done
clear
C)
pulsation theory
done
clear
D)
Transpiration pull
done
clear
View Answer play_arrow
question_answer 168) Which enzyme converts glucose into alcohol?
A)
Zymase
done
clear
B)
Diastase
done
clear
C)
Invertase
done
clear
D)
Lipase
done
clear
View Answer play_arrow
question_answer 169) Which of the following is not the feature of gymnosperms?
A)
Parallel ventation
done
clear
B)
Perennial plants
done
clear
C)
Distinct branches (long and short branches)
done
clear
D)
Xylem with vessels
done
clear
View Answer play_arrow
question_answer 170) Which of the following is important for muscle contraction and nerve impulse transmission?
A)
\[C{{a}^{2+}}ions\]
done
clear
B)
\[M{{g}^{2+}}ions\]
done
clear
C)
Both [a] and [b]
done
clear
D)
Fe2+ions
done
clear
View Answer play_arrow
question_answer 171) The presence of diversity at the junction of territories of two different habitats is known as
A)
bottle neck effect
done
clear
B)
edge effect
done
clear
C)
junction effect
done
clear
D)
Pasteur effect
done
clear
View Answer play_arrow
question_answer 172) In which form does the food transported in plants?
A)
Sucrose
done
clear
B)
Fructose
done
clear
C)
Glucose
done
clear
D)
Lactose
done
clear
View Answer play_arrow
question_answer 173) Lady finger belongs to family
A)
Malvaceae
done
clear
B)
Cucurbitaceae
done
clear
C)
Liliaceae
done
clear
D)
Brassicaceae
done
clear
View Answer play_arrow
question_answer 174) In Cycas, pollination takes place in
A)
3 celled stage
done
clear
B)
4 celled stage
done
clear
C)
2 celled stage
done
clear
D)
1 celled stage
done
clear
View Answer play_arrow
question_answer 175) The bioassay of auxin is
A)
avena curvature test
done
clear
B)
callus formation
done
clear
C)
culture of fungus
done
clear
D)
seed dormancy
done
clear
View Answer play_arrow
question_answer 176) Which of these statements about Huntingtons disease is true?
A)
Genetic tests to detect the presence of the allele responsible for Huntingtons disease do not exist at this time
done
clear
B)
The onset of Huntingtons disease is typically between birth and three year of age
done
clear
C)
There is currently no effective treatment of Huntingtons disease
done
clear
D)
Huntingtons disease is caused by the expression of a recessive allele
done
clear
View Answer play_arrow
question_answer 177) Which one is component of ornithine cycle?
A)
Ornithine, citrulline and alanine
done
clear
B)
Ornithine, citrulline and arginine
done
clear
C)
Amino acid are not used
done
clear
D)
Ornithine, citrulline and fumaric acid
done
clear
View Answer play_arrow
question_answer 178) Chromosome complement with \[2n-1\] is called
A)
monosomy
done
clear
B)
nullisomy
done
clear
C)
trisomy
done
clear
D)
tetrasomy
done
clear
View Answer play_arrow
question_answer 179) Energy transferred from one trophic level to another is
A)
5%
done
clear
B)
10%
done
clear
C)
15%
done
clear
D)
20%
done
clear
View Answer play_arrow
question_answer 180) Small fish get stuck near the bottom of a shark and derives its nutrition from it. This kind of association is called as
A)
antibiosis
done
clear
B)
commensalism
done
clear
C)
predation
done
clear
D)
parasitism
done
clear
View Answer play_arrow
question_answer 181) Which of the following is not vestigial in man?
A)
Tail vertebrae
done
clear
B)
Nails
done
clear
C)
Nictitating membrane
done
clear
D)
Vermiform appendix
done
clear
View Answer play_arrow
question_answer 182) A eukaryotic gene contains two kinds of base sequences. Which of these plays an important role in protein synthesis?
A)
Introns
done
clear
B)
Exons
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 183) The number of hydrogen bonds between adenine and thymine in a DNA molecule is
A)
two
done
clear
B)
three
done
clear
C)
four
done
clear
D)
eight
done
clear
View Answer play_arrow
question_answer 184) The group of anamniota includes
A)
reptiles and birds
done
clear
B)
birds and mammals
done
clear
C)
fishes an amphibians
done
clear
D)
reptiles and mammals
done
clear
View Answer play_arrow
question_answer 185) The excretory material of bony fish is
A)
urea
done
clear
B)
protein
done
clear
C)
ammonia
done
clear
D)
amino acid
done
clear
View Answer play_arrow
question_answer 186) The leucocytes contain which of the following in large quantity?
A)
Basophils
done
clear
B)
Neutrophils
done
clear
C)
Eosinophils
done
clear
D)
Monocytes
done
clear
View Answer play_arrow
question_answer 187) During inspiration, the diaphragm
A)
expands
done
clear
B)
shows no change
done
clear
C)
contracts and flattens
done
clear
D)
relaxes to become dome-shaped
done
clear
View Answer play_arrow
question_answer 188) The function of pineal body is to
A)
lighten the skin colours
done
clear
B)
control sexual behaviour
done
clear
C)
regulates the period of puberty
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 189) Synsacrum of fowl consists of about
A)
29 vertebrae
done
clear
B)
3 vertebrae
done
clear
C)
16 vertebrae
done
clear
D)
single vertebrae
done
clear
View Answer play_arrow
question_answer 190) Which of the following was formed in S Millers experiment?
A)
Amino acids
done
clear
B)
Nucleic acids
done
clear
C)
UV radiations
done
clear
D)
Microspheres
done
clear
View Answer play_arrow
question_answer 191) Which of the following variations are temporary and have nothing to do with the last or next generation?
A)
Hereditary variations
done
clear
B)
Discontinuous variations
done
clear
C)
Environmental variations
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 192) The highest cranial capacity is/was present in
A)
Java man
done
clear
B)
Peking man
done
clear
C)
Handy man
done
clear
D)
Modern man
done
clear
View Answer play_arrow
question_answer 193) A marriage between normal visioned man and colourblind woman will produce which of the following types of offspring?
A)
Normal sons and carrier daughters
done
clear
B)
Colourblind sons and carrier daughters
done
clear
C)
Colourblind sons and 50% carrier daughters
done
clear
D)
50% colourblind sons and 50% carrier daughters
done
clear
View Answer play_arrow
question_answer 194) L-shaped chromosomes are also called
A)
acrocentric
done
clear
B)
telocentric
done
clear
C)
sub-metacentric
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 195) Which of the following is/are grouped under phanerogams?
A)
Angiosperms
done
clear
B)
Gymnosperms
done
clear
C)
Pteriodophytes
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 196) Assertion The imbalance in concentration of \[N{{a}^{+}},{{K}^{+}}\]and proteins generates resting potential. Reason To maintain the unequal distribution of \[N{{a}^{+}}\]and \[{{K}^{+}},\]the neurons use electrical energy.
A)
If both Assertion and Reason are true and Reason is the correct explanation of the Assertion.
done
clear
B)
If both Assertion and Reason are true but the Reason is not the correct explanations of Assertion.
done
clear
C)
If Assertion is true, but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 197) Assertion A coenzymes of metal ions that is very tightly bound to enzyme protein is called prosthetic group. Reason A complete, catalytically active enzyme together with its bound prosthetic group is called apoenzyme.
A)
If both Assertion and Reason are true and Reason is the correct explanation of the Assertion.
done
clear
B)
If both Assertion and Reason are true but the Reason is not the correct explanations of Assertion.
done
clear
C)
If Assertion is true, but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 198) Assertion In cymose tap root system, oldest branch lies very near the growing point of the root, while the youngest branch is farthest away from it. Reason In cymose tap root system, the primary root itself stops growing after some
A)
If both Assertion and Reason are true and Reason is the correct explanation of the Assertion.
done
clear
B)
If both Assertion and Reason are true but the Reason is not the correct explanations of Assertion.
done
clear
C)
If Assertion is true, but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 199) Assertion Cyclic pathway of photosynthesis first appeared in some eubacterial species. Reason Oxygen started accumulating in the atmosphere after the non-cyclic pathway of photosynthesis evolved,
A)
If both Assertion and Reason are true and Reason is the correct explanation of the Assertion.
done
clear
B)
If both Assertion and Reason are true but the Reason is not the correct explanations of Assertion.
done
clear
C)
If Assertion is true, but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
question_answer 200) Assertion Mast cells in the human body release excessive amount of inflammatory chemicals, which cause allergic reactions, Reason Allergens in the environment on reacting human body stimulate mast cells in certain individuals,
A)
If both Assertion and Reason are true and Reason is the correct explanation of the Assertion.
done
clear
B)
If both Assertion and Reason are true but the Reason is not the correct explanations of Assertion.
done
clear
C)
If Assertion is true, but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow