A) \[B{{F}_{3}}<{{H}_{2}}S<{{H}_{2}}O\]
B) \[{{H}_{2}}S<B{{F}_{3}}<{{H}_{2}}O\]
C) \[{{H}_{2}}O<{{H}_{2}}S<B{{F}_{3}}\]
D) \[{{H}_{2}}O<B{{F}_{3}}<{{H}_{2}}S\]
Correct Answer: A
Solution :
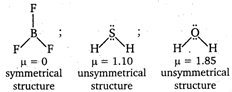 Oxygen is more electronegative than sulphur. Hence, the order of dipole moment is as \[B{{F}_{3}}<{{H}_{2}}S<{{H}_{2}}O\]
Oxygen is more electronegative than sulphur. Hence, the order of dipole moment is as \[B{{F}_{3}}<{{H}_{2}}S<{{H}_{2}}O\]
You need to login to perform this action.
You will be redirected in
3 sec
