R.Q. is the ratio of the volume of \[C{{O}_{2}}\]released to the volume of oxygen taken in respiration and is written as
\[R.Q.=\frac{Volume\,of\,C{{O}_{2}}\,evolved}{Volume\,of\,{{O}_{2}}\,absorbed}=\frac{C{{O}_{2}}}{{{O}_{2}}}\]
R.Q. is usually measured by Ganong's respirometer.
(1) When carbohydrates are the respiratory substrate (=germinating wheat, oat, barley, paddy grains or green leaves kept in dark or tubers, rhizomes, etc.)
\[\underset{\text{Glucose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,+6{{O}_{2}}\to 6C{{O}_{2}}+6{{H}_{2}}O;\,\,\frac{C{{O}_{2}}}{{{O}_{2}}}=\frac{6}{6}=1\] (Unity)
(2) When fats are the respiratory substrate (=germinating castor, mustard, linseed, til seeds) for fatty substances R.Q. is generally less than one .
(i) \[\underset{\text{Stearic}\,\text{acid}}{\mathop{{{C}_{18}}{{H}_{36}}{{O}_{2}}}}\,+26{{O}_{2}}\to 18C{{O}_{2}}+18{{H}_{2}}O;\frac{C{{O}_{2}}}{{{O}_{2}}}=\frac{18}{26}=0.7\](Less than unity)
(ii) \[\underset{\text{Tripalmitin}}{\mathop{2{{C}_{51}}{{H}_{98}}{{O}_{6}}}}\,+145{{O}_{2}}\to 102C{{O}_{2}}+98{{H}_{2}}O;\frac{C{{O}_{2}}}{{{O}_{2}}}=\frac{102}{145}=0.7\] (Less than unity)
(3) When protein are the respiratory substrate (=germinating gram, pea, bean, mung seeds)
value of R.Q. is less than unity (0.5-0.9).
(4) When organic acids are the respiratory substrate
(i)\[\underset{\text{Malic}\,\text{acid}}{\mathop{{{C}_{4}}{{H}_{6}}{{O}_{5}}}}\,+3{{O}_{2}}\to 4C{{O}_{2}}+3{{H}_{2}}O;\frac{C{{O}_{2}}}{{{O}_{2}}}=\frac{4}{3}=1.33\] (More than unity)
(ii) \[\underset{\text{Oxalic}\,\text{acid}}{\mathop{2{{(COOH)}_{2}}}}\,+{{O}_{2}}\to 4C{{O}_{2}}+2{{H}_{2}}O;\frac{C{{O}_{2}}}{{{O}_{2}}}=\frac{4}{1}=4\] (More than unity)
Some other organic acids and their R.Q. are – Succinic acid (1.14), Taurtric acid (1.6) and Acetic acid (1).
(5) When
more...
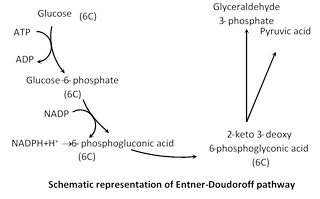 (2) Pentose phosphate pathway
(i) Discovery : It is also called as Hexose monophosphate (HMP) shunt or Warburg Dickens pathway or direct oxidation pathway. It provides as alternative pathway for breakdown of glucose which is independent of EMP pathway (glycolysis) and Krebs cycle. Its existence was suggested for the first time by Warburg et al. (1935) and Dickens (1938). Most of the reaction of this cycle were described by Horecker et al. (1951) and Racker (1954).
(ii) Occurrence : Pentose phosphate pathway that exists in many organisms. This pathway takes more...
(2) Pentose phosphate pathway
(i) Discovery : It is also called as Hexose monophosphate (HMP) shunt or Warburg Dickens pathway or direct oxidation pathway. It provides as alternative pathway for breakdown of glucose which is independent of EMP pathway (glycolysis) and Krebs cycle. Its existence was suggested for the first time by Warburg et al. (1935) and Dickens (1938). Most of the reaction of this cycle were described by Horecker et al. (1951) and Racker (1954).
(ii) Occurrence : Pentose phosphate pathway that exists in many organisms. This pathway takes more...
