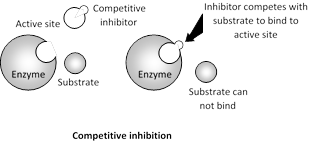
 The concentration of \[EI\]complex depends on the concentration of free inhibitor. Because \[EI\]complex readily dissociates, the empty active sites are then available for substrate binding. The effect of a competitive inhibitor on activity is reversed by increasing the concentration of substrate. In it \[{{V}_{\max }}\] remain constant and Km increases.
A classic example of competitive inhibition is succinic acid dehydrogenase which oxidises succinic acid to fumaric acid. If concentration of malonic more...
The concentration of \[EI\]complex depends on the concentration of free inhibitor. Because \[EI\]complex readily dissociates, the empty active sites are then available for substrate binding. The effect of a competitive inhibitor on activity is reversed by increasing the concentration of substrate. In it \[{{V}_{\max }}\] remain constant and Km increases.
A classic example of competitive inhibition is succinic acid dehydrogenase which oxidises succinic acid to fumaric acid. If concentration of malonic more...
You need to login to perform this action.
You will be redirected in
3 sec
