question_answer 1) The only mechanical quantity which has negative dimension of mass is
A)
angular momentum
done
clear
B)
torque
done
clear
C)
coefficient of thermal conductivity
done
clear
D)
gravitational constant
done
clear
View Answer play_arrow
question_answer 2) Two vectors are given by \[\vec{A}=3\hat{i}+\hat{j}+3\hat{k}\]and \[\vec{B}=3\hat{i}+5\hat{j}-2\hat{k}\]Find the third vector \[\hat{C}\] if \[\vec{A}+3\vec{B}-\vec{C}=\vec{O}\]
A)
\[12\vec{i}+14\hat{j}+12\hat{k}\]
done
clear
B)
\[13\hat{i}+17\hat{j}+12\hat{k}\]
done
clear
C)
\[12\hat{i}+16\hat{j}-3\hat{k}\]
done
clear
D)
\[15\hat{i}+13\hat{j}+4\hat{k}\]
done
clear
View Answer play_arrow
question_answer 3) A particle moves from position \[3\hat{i}+2\hat{j}+6\hat{k}\]to \[14\hat{i}+13\hat{j}+9\hat{k}\]due to a uniform force of \[4\hat{i}+\hat{j}+3\hat{k}\] N. Find the work done, if the displacement is in metre.
A)
16 J
done
clear
B)
64 J
done
clear
C)
32 J
done
clear
D)
48 J
done
clear
View Answer play_arrow
question_answer 4) The coordinates of a moving particle at any time t are given by \[x=\alpha {{t}^{3}}\] and \[y=\beta {{t}^{3}}\]. The speed of the particle at time t is given by
A)
\[3t\sqrt{{{\alpha }^{2}}+{{\beta }^{2}}}\]
done
clear
B)
\[3{{t}^{2}}\sqrt{{{\alpha }^{2}}+{{\beta }^{2}}}\]
done
clear
C)
\[{{t}^{2}}\sqrt{{{\alpha }^{2}}+{{\beta }^{2}}}\]
done
clear
D)
\[\sqrt{{{\alpha }^{2}}+{{\beta }^{2}}}\]
done
clear
View Answer play_arrow
question_answer 5) A body is moving with uniform acceleration covers 200 m in the first 2 s and 220 m in the next 4 s. Find the velocity in m/s after 7 s.
A)
10
done
clear
B)
15
done
clear
C)
20
done
clear
D)
30
done
clear
View Answer play_arrow
question_answer 6) From the top of a tower a body A is projected vertically up, another body B is horizontally thrown and a third body C is thrown vertically down with same velocity. Then
A)
B strikes the ground with more velocity
done
clear
B)
C strikes the ground with less velocity
done
clear
C)
A, B, C strike the ground with same velocity
done
clear
D)
A and C strike the ground with more velocity than B
done
clear
View Answer play_arrow
question_answer 7) A body is released from a great height falls freely towards the earth. Another body is released from the same height exactly a second latter. Then the separation between two bodies, 2 s after the release of the second body is, nearly
A)
15 m
done
clear
B)
20 m
done
clear
C)
25 m
done
clear
D)
30 m
done
clear
View Answer play_arrow
question_answer 8) A body of mass 10 kg is acted upon by two forces each of magnitude 10N making an angle of 60° with each other. Find the net acceleration of the body.
A)
\[2\sqrt{3}m/{{s}^{2}}\]
done
clear
B)
\[\sqrt{3}m/{{s}^{2}}\]
done
clear
C)
\[3\sqrt{3}m/{{s}^{2}}\]
done
clear
D)
\[4\sqrt{3}m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 9) A body of mass \[{{m}_{1}}\] collides elastically with another body of mass \[{{m}_{2}}\] at rest. If the velocity of \[{{m}_{1}}\] after collision becomes 2/3 times its initial velocity, the ratio of their masses, is
A)
1 : 5
done
clear
B)
5 : 1
done
clear
C)
5 : 2
done
clear
D)
2 : 5
done
clear
View Answer play_arrow
question_answer 10) With increase of temperature, the frictional force acting between two surfaces
A)
increases
done
clear
B)
remains the same
done
clear
C)
decreases
done
clear
D)
becomes zero
done
clear
View Answer play_arrow
question_answer 11) The motion of the centre of mass is the result of
A)
internal forces
done
clear
B)
external forces
done
clear
C)
attractive forces
done
clear
D)
repulsive forces
done
clear
View Answer play_arrow
question_answer 12) A stationary bomb explodes into two parts of masses in the ratio of 1 : 3. If the heavier mass moves with a velocity 4 m/s, what is the velocity of lighter part?
A)
\[12\text{ }m{{s}^{-1}}\]opposite to heavier mass
done
clear
B)
\[12\text{ }m{{s}^{-1}}\] in the direction of heavier mass
done
clear
C)
\[6\text{ }m{{s}^{-1}}\]opposite to heavier mass
done
clear
D)
\[6\text{ }m{{s}^{-1}}\]in the direction of heavier mass
done
clear
View Answer play_arrow
question_answer 13) A body is tied to one end of the string and whirled in a vertical circle, the physical quantity which remains constant is
A)
momentum
done
clear
B)
speed
done
clear
C)
kinetic energy
done
clear
D)
total energy
done
clear
View Answer play_arrow
question_answer 14) A thin metal disc of radius of 0.25 m and mass 2 kg starts from rest and rolls down on an inclined plane. If its rotational kinetic energy is 4 J at the foot of inclined plane, then the linear velocity at the same point, is in m/s
A)
2
done
clear
B)
\[2\sqrt{2}\]
done
clear
C)
\[2\sqrt{3}\]
done
clear
D)
\[3\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 15) A stone tied to one end of rope and rotated in a circular motion. If the string suddenly breaks, then the stone travels
A)
in perpendicular direction
done
clear
B)
in direction of centrifugal force
done
clear
C)
towards centripetal force
done
clear
D)
in tangential direction
done
clear
View Answer play_arrow
question_answer 16) The escape velocity of a body from the earth is Ve. If the radius of earth contracts to \[\frac{1}{4}\] th of its value, keeping the mass of the earth constant, the escape velocity will be
A)
doubled
done
clear
B)
halved
done
clear
C)
tripled
done
clear
D)
unaltered
done
clear
View Answer play_arrow
question_answer 17) The radius of the earth is R. The height of a point vertically above the earths surface at which acceleration due to gravity becomes 1% of its value at the surface is
A)
8R
done
clear
B)
9R
done
clear
C)
10R
done
clear
D)
20R
done
clear
View Answer play_arrow
question_answer 18) A heavy uniform rod is hanging vertically from a fixed support. It is stretched by its own weight. The diameter of the rod is
A)
smallest at the top and gradually increases down the rod
done
clear
B)
largest at the top and gradually decreases down the rod
done
clear
C)
uniform everywhere
done
clear
D)
maximum in the middle
done
clear
View Answer play_arrow
question_answer 19) A particular force (F) applied on a wire increases its length by \[2\times {{10}^{-3}}m.\]To increase the wires length by \[4\times {{10}^{-3}}\] m the applied force will be
A)
4F
done
clear
B)
3F
done
clear
C)
2F
done
clear
D)
F
done
clear
View Answer play_arrow
question_answer 20) The meniscus of mercury in a capillary glass tube, is
A)
concave
done
clear
B)
plane
done
clear
C)
cylindrical
done
clear
D)
convex
done
clear
View Answer play_arrow
question_answer 21) Two liquid drops have diameters of 1 cm and 1.5 cm. The ratio of excess of pressures inside them is
A)
1 : 1
done
clear
B)
5 : 3
done
clear
C)
2 : 3
done
clear
D)
3 : 2
done
clear
View Answer play_arrow
question_answer 22) Temperature remaining constant, the pressure of gas is decreased by 20%. The percentage change in volume
A)
increases by 20%
done
clear
B)
decreases by 20%
done
clear
C)
increases by 25%
done
clear
D)
decreases by 25%
done
clear
View Answer play_arrow
question_answer 23) Work done in converting one gram of ice at \[-10{}^\circ C\] into steam at \[100{}^\circ C\] is
A)
3045 J
done
clear
B)
6056 J
done
clear
C)
721 J
done
clear
D)
616 J
done
clear
View Answer play_arrow
question_answer 24) The temperature of the system decreases in the process of
A)
free expansion
done
clear
B)
adiabatic expansion
done
clear
C)
isothermal expansion
done
clear
D)
isothermal compression
done
clear
View Answer play_arrow
question_answer 25) The temperature at which a black body ceases to radiate energy, is
A)
0K
done
clear
B)
273 K
done
clear
C)
30 K
done
clear
D)
100 K
done
clear
View Answer play_arrow
question_answer 26) A particle executes SHM with a period of 8 s and amplitude 4 cm. Its maximum speed in cm/s, is
A)
\[\pi \]
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
\[\frac{\pi }{3}\]
done
clear
D)
\[\frac{\pi }{4}\]
done
clear
View Answer play_arrow
question_answer 27) The displacement of a particle of mass 3 g executing simple harmonic motion is given by y = 3 sin (0.20 in SI units. The KE of the particle at a point which is at a distance equal to 1/3 of its amplitude from its mean position is
A)
\[12\times {{10}^{-3}}J\]
done
clear
B)
\[25\times {{10}^{-3}}J\]
done
clear
C)
\[0.48\times {{10}^{-3}}J\]
done
clear
D)
\[0.24\times {{10}^{-3}}J\]
done
clear
View Answer play_arrow
question_answer 28) A uniform spring of force constant k is cut into two pieces whose lengths are in the ratio of 1:2. What is the force constant of second piece in terms of k?
A)
\[\frac{k}{2}\]
done
clear
B)
\[\frac{2k}{2}\]
done
clear
C)
\[\frac{3k}{2}\]
done
clear
D)
\[\frac{4k}{2}\]
done
clear
View Answer play_arrow
question_answer 29) A girl swings on cradle in a sitting position. If she stands what happens to the time period of girl and cradle?
A)
Time period decreases
done
clear
B)
Time period increases
done
clear
C)
Remains constant
done
clear
D)
First increases and then remains constant
done
clear
View Answer play_arrow
question_answer 30) The intensity ratio of two waves is 1 : 9. The ratio of their amplitudes, is
A)
3 : 1
done
clear
B)
1 : 3
done
clear
C)
1 : 9
done
clear
D)
9 : 1
done
clear
View Answer play_arrow
question_answer 31) The speed of sound waves in a gas
A)
does not depend upon density of the gas
done
clear
B)
does not depend upon changes in pressure
done
clear
C)
does not depend upon temperature
done
clear
D)
depends upon density of the gas
done
clear
View Answer play_arrow
question_answer 32) A segment of wire vibrates with fundamental frequency of 450 Hz under a tension of 9 kg-wt. Then tension at which the fundamental frequency of the same wire becomes 900 Hz is
A)
36 kg-wt
done
clear
B)
27 kg-wt
done
clear
C)
18 kg-wt
done
clear
D)
72 kg-wt
done
clear
View Answer play_arrow
question_answer 33) Sound waves in air cannot be polarized because
A)
their speed is small
done
clear
B)
they require medium
done
clear
C)
these are longitudinal
done
clear
D)
their speed is temperature dependent
done
clear
View Answer play_arrow
question_answer 34) Electric potential at the centre of a charged hollow metal sphere is
A)
zero
done
clear
B)
twice as that on the surface
done
clear
C)
half of that on the surface
done
clear
D)
same as that on the surface
done
clear
View Answer play_arrow
question_answer 35) Three charges \[1\mu C,\,1\mu C\]and \[2\mu C\] are kept at vertices of A, B and C of an equilateral triangle ABC of 10 cm side respectively. The resultant force on the charge at C is
A)
0.9 N
done
clear
B)
1.8 N
done
clear
C)
2.72 N
done
clear
D)
3.12 N
done
clear
View Answer play_arrow
question_answer 36) A dielectric of dielectric constant K is introduced such that half of its area of a capacitor of capacity C is occupied by it. The new capacity is
A)
2 C
done
clear
B)
C/2
done
clear
C)
(1 +k) C/2
done
clear
D)
2C (1 + k)
done
clear
View Answer play_arrow
question_answer 37) The value equal to the velocity of light in vacuum is
A)
\[\frac{\sqrt{{{\mu }_{0}}}}{{{\varepsilon }_{0}}}\]
done
clear
B)
\[\frac{1}{\sqrt{{{\mu }_{0}}{{\varepsilon }_{0}}}}\]
done
clear
C)
\[\sqrt{{{\mu }_{0}}{{\varepsilon }_{0}}}\]
done
clear
D)
\[\sqrt{\frac{{{\mu }_{0}}}{{{\varepsilon }_{0}}}}\]
done
clear
View Answer play_arrow
question_answer 38) Two unlike charges of the same magnitude Q are placed at a distance d. The intensity of the electric field at the middle point in the line joining the two charges
A)
zero
done
clear
B)
\[\frac{8Q}{4\pi {{\varepsilon }_{0}}{{d}^{2}}}\]
done
clear
C)
\[\frac{6Q}{4\pi {{\varepsilon }_{0}}{{d}^{2}}}\]
done
clear
D)
\[\frac{4Q}{4\pi {{\varepsilon }_{0}}{{d}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 39) In a meter bridge experiment, the ratio of the left gap resistance to right gap resistance is 2 : 3, the balance point from left is
A)
60 cm
done
clear
B)
50 cm
done
clear
C)
40 cm
done
clear
D)
20 cm
done
clear
View Answer play_arrow
question_answer 40) The physical quantity in electrostatics analogous to temperature in heat is
A)
heat energy
done
clear
B)
capacity
done
clear
C)
resistance
done
clear
D)
potential
done
clear
View Answer play_arrow
question_answer 41) If the electric current through an electric bulb is 3.2 A, the number of electrons flow through it in one second is
A)
\[2\times {{10}^{9}}\]
done
clear
B)
\[2\times {{10}^{19}}\]
done
clear
C)
\[3.2\times {{10}^{19}}\]
done
clear
D)
\[1.6\times {{10}^{18}}\]
done
clear
View Answer play_arrow
question_answer 42) A wire P has a resistance of 20 \[\Omega \] Another wire Q of same material but length twice that of P has resistance of 8 \[\Omega \] If r is the radius of cross-section of P, the radius of cross-section of Q is
A)
r
done
clear
B)
\[\frac{r}{\sqrt{2}}\]
done
clear
C)
\[\sqrt{5}\,\,r\]
done
clear
D)
2r
done
clear
View Answer play_arrow
question_answer 43) When a metal conductor connected to the left gap of a meter bridge is heated, the balancing point
A)
shifts towards right
done
clear
B)
shifts towards left
done
clear
C)
remains unchanged
done
clear
D)
remains at zero
done
clear
View Answer play_arrow
question_answer 44) The thermocouple among the following that can produce maximum thermo emf for the same temperature difference between the junction is
A)
Fe-Cu
done
clear
B)
Ag-Au
done
clear
C)
Sb-Bi
done
clear
D)
Cu-Pb
done
clear
View Answer play_arrow
question_answer 45) A bar magnet of magnetic moment M and moment of inertia I is freely suspended such that the magnetic axial line is in the direction of magnetic meridian. If the magnet is displaced by a very small angle \[\theta \], angular acceleration is (magnetic induction of earths horizontal field\[={{B}_{H}}\])
A)
\[\frac{M{{B}_{H}}\theta }{1}\]
done
clear
B)
\[\frac{I{{B}_{H}}\theta }{M}\]
done
clear
C)
\[\frac{M\theta }{I{{B}_{H}}}\]
done
clear
D)
\[\frac{I\theta }{M{{B}_{H}}}\]
done
clear
View Answer play_arrow
question_answer 46) A long magnet is cut into two equal parts, such that the length of each half is same as that of original magnet. If the period of original magnet is T, the period of new magnet is
A)
T
done
clear
B)
\[\frac{T}{2}\]
done
clear
C)
\[\frac{T}{4}\]
done
clear
D)
2T
done
clear
View Answer play_arrow
question_answer 47) The time period of a freely suspended bar magnet in a field is 2 s. It is cut into two equal parts along its axis, then the time period is
A)
4 s
done
clear
B)
0.5 s
done
clear
C)
2 s
done
clear
D)
0.25 s
done
clear
View Answer play_arrow
question_answer 48) The angle between magnetic meridian and geographical meridian is known as
A)
magnetic dip
done
clear
B)
magnetic latitude
done
clear
C)
magnetic declination
done
clear
D)
magnetic longitude
done
clear
View Answer play_arrow
question_answer 49) The net magnetic flux through any closed surface, kept in a magnetic field is
A)
zero
done
clear
B)
\[\frac{{{\mu }_{0}}}{4\pi }\]
done
clear
C)
\[4\pi {{\mu }_{0}}\]
done
clear
D)
\[\frac{4{{\mu }_{0}}}{\pi }\]
done
clear
View Answer play_arrow
question_answer 50) A paramagnetic liquid is taken in a U-tube and arranged so that one of its limbs is kept between pole pieces of the magnet. The liquid level in the limb
A)
goes down
done
clear
B)
rises up
done
clear
C)
remains same
done
clear
D)
first goes down and then rises
done
clear
View Answer play_arrow
question_answer 51) Two wires A and B are of lengths 40 cm and 30 cm. A is bent into a circle of radius r and B into an arc of radius r. A current \[{{i}_{1}}\]is passed through A and \[{{i}_{2}}\]through B. To have the same magnetic inductions at the centre, the ratio of \[{{i}_{1}}:\text{ }{{i}_{2}}\]is
A)
3 : 4
done
clear
B)
3 : 5
done
clear
C)
2 : 3
done
clear
D)
4 : 3
done
clear
View Answer play_arrow
question_answer 52) An electron and proton having same kinetic energy enter into magnetic field perpendicular to it. Then
A)
the path of electron is less curved
done
clear
B)
the path of proton is less curved
done
clear
C)
both have equal curved paths
done
clear
D)
both have straight line paths
done
clear
View Answer play_arrow
question_answer 53) Two free parallel wires carrying currents in the opposite directions
A)
attract each other
done
clear
B)
repel each other
done
clear
C)
do not effect each other
done
clear
D)
get rotated to be perpendicular to each other
done
clear
View Answer play_arrow
question_answer 54) A circular coil of diameter 21 cm is placed in a magnetic field of induction \[{{10}^{-4}}T.\] The magnitude of flux linked with coil when the plane of coil makes an angle 30° with the field is
A)
\[1.44\times {{10}^{-6}}Wb\]
done
clear
B)
\[1.732\times {{10}^{-6}}Wb\]
done
clear
C)
\[3.1\times l{{0}^{-6}}Wb\]
done
clear
D)
\[4.2\times {{10}^{-6}}Wb\]
done
clear
View Answer play_arrow
question_answer 55) The cladding material of optical fibre has refractive index
A)
greater than that of core
done
clear
B)
infinity
done
clear
C)
equal to that of core
done
clear
D)
less than that of core
done
clear
View Answer play_arrow
question_answer 56) Red colour is used for danger signals because
A)
it causes fear
done
clear
B)
it undergoes least scattering
done
clear
C)
it undergoes maximum scattering
done
clear
D)
it is in accordance with international convention
done
clear
View Answer play_arrow
question_answer 57) The least angle of deviation for a glass prism is equal to its refracting angle. The refractive index of glass is 1.5. Then the angle of prism is
A)
\[2{{\cos }^{-1}}\left( \frac{3}{4} \right)\]
done
clear
B)
\[{{\sin }^{-1}}\left( \frac{3}{4} \right)\]
done
clear
C)
\[2{{\sin }^{-1}}\left( \frac{3}{2} \right)\]
done
clear
D)
\[{{\cos }^{-1}}\left( \frac{3}{2} \right)\]
done
clear
View Answer play_arrow
question_answer 58) In a spectrometer experiment the prisms A B, C with same angle but different refractive index \[{{\mu }_{A}}\]= 1.33, \[{{\mu }_{B}}\]= 1.5, \[{{\mu }_{C}}\]= 1.44 are used. The corresponding angles of minimum deviation \[{{D}_{A}},\text{ }{{D}_{B}},\text{ }{{D}_{C}}\]measured will be such that
A)
\[{{D}_{A}}>\text{ }{{D}_{B}}>\text{ }{{D}_{c}}\]
done
clear
B)
\[{{D}_{A}}<\text{ }{{D}_{B}}<\text{ }{{D}_{c}}\]
done
clear
C)
\[{{D}_{A}}<\text{ }{{D}_{c}}<\text{ }{{D}_{B}}\]
done
clear
D)
\[{{D}_{A}}>\text{ }{{D}_{c}}>\text{ }{{D}_{B}}\]
done
clear
View Answer play_arrow
question_answer 59) Youngs double slit experiment arrangement is shifted from air to water medium, the fringe width
A)
increases
done
clear
B)
decreases
done
clear
C)
becomes infinite
done
clear
D)
remains the same
done
clear
View Answer play_arrow
question_answer 60) On introducing a thin film in the path of one of the two interfering beams, the central fringe will shift by one fringe width. If\[\mu \] = 1.5, the thickness of the film is (wavelength of monochromatic light is \[\lambda \])
A)
4\[\lambda \]
done
clear
B)
3\[\lambda \]
done
clear
C)
2\[\lambda \]
done
clear
D)
\[\lambda \]
done
clear
View Answer play_arrow
question_answer 61) The critical angle of the medium with respect to vacuum is 30°. If the velocity of light in vacuum is \[3\times {{10}^{-8}}m{{s}^{-1}},\] the velocity of light in medium is
A)
\[2\times {{10}^{-8}}m{{s}^{-1}}\]
done
clear
B)
\[1.5\times {{10}^{8}}m{{s}^{-1}}\]
done
clear
C)
\[3\times {{10}^{8}}m{{s}^{-1}}\]
done
clear
D)
\[\sqrt{2}\times {{10}^{8}}m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 62) The distance between the first dark and bright band formed in Youngs double slit experiment with band width B is
A)
\[\frac{B}{4}\]
done
clear
B)
B
done
clear
C)
\[\frac{B}{2}\]
done
clear
D)
\[\frac{3B}{2}\]
done
clear
View Answer play_arrow
question_answer 63) The important conclusion given by Millikans experiment about the charge is
A)
charge is never quantized
done
clear
B)
charge has no definite value
done
clear
C)
charge is quantized
done
clear
D)
charge on oil drop always increases
done
clear
View Answer play_arrow
question_answer 64) An electron is accelerated through a potential difference of V volts. The speed of electrons will be
A)
\[\sqrt{\frac{eV}{m}}\]
done
clear
B)
\[\sqrt{\frac{2eV}{m}}\]
done
clear
C)
\[\sqrt{\frac{eV}{2m}}\]
done
clear
D)
\[\sqrt{\frac{m}{2eV}}\]
done
clear
View Answer play_arrow
question_answer 65) How many photons are emitted by a laser source of \[5\times {{10}^{-3}}W\]operating at 632.2 nm in 2s?
A)
\[3.2\times {{10}^{16}}\]
done
clear
B)
\[1.6\times {{10}^{16}}\]
done
clear
C)
\[4\times {{10}^{16}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 66) If a is radius of first Bohr orbit in hydrogen atom, the radius of the third orbit is
A)
3a
done
clear
B)
9a
done
clear
C)
27a
done
clear
D)
81a
done
clear
View Answer play_arrow
question_answer 67) Nuclear fission can be explained based on
A)
Millikans oil drop method
done
clear
B)
Liquid drop model
done
clear
C)
Shell model
done
clear
D)
Bohrs model
done
clear
View Answer play_arrow
question_answer 68) The radius of a nucleus with atomic mass number 7 is 2 Fermi. Find the radius of nucleus with atomic number 189.
A)
3 Fermi
done
clear
B)
4 Fermi
done
clear
C)
5 Fermi
done
clear
D)
6 Fermi
done
clear
View Answer play_arrow
question_answer 69) As mass number increases, surface area
A)
decreases
done
clear
B)
increases
done
clear
C)
remains the same
done
clear
D)
remains the same and increases
done
clear
View Answer play_arrow
question_answer 70) All nucleons in an atom are held by
A)
nuclear forces
done
clear
B)
van der Waals forces
done
clear
C)
tensor forces
done
clear
D)
Coloumb forces
done
clear
View Answer play_arrow
question_answer 71) The presence of electric charge on colloidal particles is indicated by the property, called
A)
dialysis
done
clear
B)
solubility
done
clear
C)
electrophoresis
done
clear
D)
osmosis
done
clear
View Answer play_arrow
question_answer 72) The protective action of different lyophilic colloids is expressed in terms of
A)
oxidation number
done
clear
B)
atomic number
done
clear
C)
Avogadro number
done
clear
D)
gold number
done
clear
View Answer play_arrow
question_answer 73) The group of elements in which the differentiating electron enters the anti penultimate shell of atoms are called
A)
\[f-\]block elements
done
clear
B)
p-block elements
done
clear
C)
\[s-\]block elements
done
clear
D)
d-block elements
done
clear
View Answer play_arrow
question_answer 74) Lanthanides and actinides are also called as
A)
short periods
done
clear
B)
inner-transition elements
done
clear
C)
long periods
done
clear
D)
main transition elements
done
clear
View Answer play_arrow
question_answer 75) The geometrical shape of\[s{{p}^{3}}d\]hybridisation is
A)
linear
done
clear
B)
trigonal bipyramid
done
clear
C)
square planar
done
clear
D)
tetrahedral
done
clear
View Answer play_arrow
question_answer 76) Inter-molecular hydrogen bonding exists in
A)
o-nitrophenol
done
clear
B)
o-chlorophenol
done
clear
C)
water
done
clear
D)
ammonium chloride
done
clear
View Answer play_arrow
question_answer 77) Which one of the following examples exhibit transient existence?
A)
H
done
clear
B)
\[H_{2}^{+}\]
done
clear
C)
\[{{H}^{+}}\]
done
clear
D)
\[He\]
done
clear
View Answer play_arrow
question_answer 78) The number of covalent bonds in fluorine molecule is
A)
2
done
clear
B)
3
done
clear
C)
1
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 79) Carbon shows the following oxidation state in its hydrides
A)
+1
done
clear
B)
+4
done
clear
C)
+2
done
clear
D)
+3
done
clear
View Answer play_arrow
question_answer 80) The following is not an example of oxyacids of sulphur
A)
\[{{H}_{2}}S{{O}_{3}}\]
done
clear
B)
\[{{H}_{2}}S{{P}_{3}}\]
done
clear
C)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
\[S{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 81) Calcium sulphate is sparingly soluble in
A)
water
done
clear
B)
alcohol
done
clear
C)
acetic acid
done
clear
D)
benzene
done
clear
View Answer play_arrow
question_answer 82) The bleaching action of chlorine is due to the liberation of the following
A)
\[HOCl\]
done
clear
B)
\[HCl\]
done
clear
C)
\[[O]\]
done
clear
D)
\[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 83) The colour of transition metal ions is due to the presence of unpaired electron transitions in available empty electron in
A)
d-orbitals
done
clear
B)
p-orbitals
done
clear
C)
s-orbitals
done
clear
D)
s and p-orbitals
done
clear
View Answer play_arrow
question_answer 84) The oxidation state of Cr in chromium trioxide is
A)
+3
done
clear
B)
+4
done
clear
C)
+5
done
clear
D)
+6
done
clear
View Answer play_arrow
question_answer 85) Monel metal is an alloy of
A)
\[Cu,\text{ }Ni,\text{ }Fe,\text{ }Mn\]
done
clear
B)
\[Cu,\text{ }Sn,\text{ }Zn\]
done
clear
C)
\[Cu,Sn,P\]
done
clear
D)
\[Cu,Zn\]
done
clear
View Answer play_arrow
question_answer 86) The catalyst used in the manufacture of \[{{H}_{2}}S{{O}_{4}}\]by contact process is
A)
\[{{V}_{2}}{{O}_{3}}\]
done
clear
B)
\[{{V}_{2}}{{O}_{5}}\]
done
clear
C)
\[FeO\]
done
clear
D)
\[Cu\]
done
clear
View Answer play_arrow
question_answer 87) The tetrahedral complexes have coordination number
A)
3
done
clear
B)
6
done
clear
C)
4
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 88) Potassium ferrocyanide is an example of
A)
tetrahedral
done
clear
B)
octahedral
done
clear
C)
square planar
done
clear
D)
linear
done
clear
View Answer play_arrow
question_answer 89) Which one amongst the following, exhibit geometrical isomerism?
A)
\[[C{{o}^{III}}{{(N{{H}_{3}})}_{5}}Br]S{{O}_{4}}\]
done
clear
B)
\[C{{o}^{III}}{{[EDTA]}^{1-}}\]
done
clear
C)
\[{{[C{{r}^{III}}{{(SCN)}_{6}}]}^{3-}}\]
done
clear
D)
\[[P{{t}^{II}}{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]
done
clear
View Answer play_arrow
question_answer 90) Benzoylacetonato beryllium exhibit isomerism of the type
A)
structural
done
clear
B)
geometrical
done
clear
C)
optical
done
clear
D)
conformational
done
clear
View Answer play_arrow
question_answer 91) The composition of malachite is
A)
\[CuFe{{S}_{2}}\]
done
clear
B)
\[CuC{{O}_{3}}\]
done
clear
C)
\[CuC{{O}_{3}}.Cu{{(OH)}_{2}}\]
done
clear
D)
\[Cu{{(OH)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 92) Which one of the following metals, is extracted on smelting of its ore in blast furnace?
A)
Iron
done
clear
B)
Sodium
done
clear
C)
Potassium
done
clear
D)
Magnesium
done
clear
View Answer play_arrow
question_answer 93) Prussian blue is obtained by mixing together aqueous solution of\[F{{e}^{3+}}\]salt with
A)
ferricyanide
done
clear
B)
ferrocyanide
done
clear
C)
hydrogen cyanide
done
clear
D)
sodium cyanide
done
clear
View Answer play_arrow
question_answer 94) The reaction intermediate produced, by homolydc cleavage of a bond is called
A)
carbene
done
clear
B)
carbocation
done
clear
C)
carbanion
done
clear
D)
free radical
done
clear
View Answer play_arrow
question_answer 95) The formation of cyanohydrin from a ketone is an example of
A)
electrophilic addition
done
clear
B)
nucleophilic substitution
done
clear
C)
nucleophilic addition
done
clear
D)
electrophilic substitution
done
clear
View Answer play_arrow
question_answer 96) According to Huckels rule an aromatic compound must possess
A)
\[(4n+1)\pi \]electrons
done
clear
B)
\[(4n+2)\pi \]electrons
done
clear
C)
\[4n\,\pi \]electrons
done
clear
D)
\[(4n+3)\pi \]electrons
done
clear
View Answer play_arrow
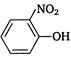
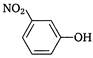
question_answer 97) Identify the substitute group, that acts as ortho-para director, during electrophilic substitution in aromatic compounds.
A)
\[N{{H}_{2}}\]
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[S{{O}_{3}}H\]
done
clear
D)
\[{{N}_{2}}\]
done
clear
View Answer play_arrow
question_answer 98) In a group of isomeric alkyl halides, the order of boiling points is
A)
primary < secondary < tertiary
done
clear
B)
primary > secondary < tertiary
done
clear
C)
primary < secondary > tertiary
done
clear
D)
primary > secondary > tertiary
done
clear
View Answer play_arrow
question_answer 99) On reacting with neutral ferric chloride, phenol gives
A)
red colour
done
clear
B)
blue colour
done
clear
C)
violet colour
done
clear
D)
green colour
done
clear
View Answer play_arrow
question_answer 100) The hybridisation of the ipso-carbon in chlorobenzene is
A)
5p hybridised
done
clear
B)
\[s{{p}^{2}}\]hybridized
done
clear
C)
\[s{{p}^{2}}d\]hybridised
done
clear
D)
\[s{{p}^{3}}\]hybridized
done
clear
View Answer play_arrow
question_answer 101) Which one of the following alcohol, is used as an antifreeze reagent for making explosives?
A)
Glycerol
done
clear
B)
Glycol
done
clear
C)
Ethanol
done
clear
D)
Phenol
done
clear
View Answer play_arrow
question_answer 102) n-pentane, 1so-pentane, and neo-pentane are examples for, isomers of the type
A)
geometrical
done
clear
B)
optical
done
clear
C)
chain
done
clear
D)
positional
done
clear
View Answer play_arrow
question_answer 103) One of the following compounds exhibit geometrical isomerism
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-HC(C{{H}_{3}})-H(C)C{{H}_{3}}-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}-HC(C{{H}_{3}})-C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}CH=CH-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 104) Different structures generated due to rotation about,\[CC\]axis, of an organic molecule, are examples of
A)
geometrical isomerism
done
clear
B)
conformational isomerism
done
clear
C)
optical isomerism
done
clear
D)
structural isomerism
done
clear
View Answer play_arrow
question_answer 105) The number of isomeric halopropanes produced, when propane gets halogenated, is
A)
1
done
clear
B)
2
done
clear
C)
\[{{4}^{o}}\]
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 106) The acid strength obeys the order. Arrange the following carboxylic acids in the decreasing order of their reactivities (1)\[C{{H}_{3}}COOH\] (2)\[ClC{{H}_{2}}COOH\] (3)\[{{C}_{12}}CHCOOH\] (4) \[C{{l}_{3}}CCOOH\]
A)
\[2>4>1>3\]
done
clear
B)
\[2>1>3>4\]
done
clear
C)
\[1>2>3>4\]
done
clear
D)
\[1<2<3<4\]
done
clear
View Answer play_arrow
question_answer 107) Monocarboxylic acids react with alcohols in the presence of an acid catalyst to form
A)
acid chlorides
done
clear
B)
acid amides
done
clear
C)
esters
done
clear
D)
ethers
done
clear
View Answer play_arrow
question_answer 108) On reaction with hydroxylamine, aldehydes produce
A)
ketoxime
done
clear
B)
hydrazine
done
clear
C)
semicarbazone
done
clear
D)
aldoxime
done
clear
View Answer play_arrow
question_answer 109) Amides are formed by the reaction of acid chloride with
A)
\[N{{H}_{2}}N{{H}_{2}}\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[N{{H}_{2}}OH\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}NHN{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 110) Which one of the following, is more acidic?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 111) Aliphatic nitriles are prepared by the treatment of alkyl halides with
A)
sodium cyanide
done
clear
B)
sodium isocyanide
done
clear
C)
sodium isocyanate
done
clear
D)
cyanamide
done
clear
View Answer play_arrow
question_answer 112) Among the following compounds, the most basic is
A)
aniline
done
clear
B)
acetanilide
done
clear
C)
p-nitroaniline
done
clear
D)
benzylamine
done
clear
View Answer play_arrow
question_answer 113) Reduction of alkyl nitriles, produces
A)
secondary amine
done
clear
B)
primary amine
done
clear
C)
tertiary amine
done
clear
D)
amide
done
clear
View Answer play_arrow
question_answer 114) Polypeptides having, molecular weights, above 10,000 are known as
A)
amino acids
done
clear
B)
harmones
done
clear
C)
proteins
done
clear
D)
terminal amino acids
done
clear
View Answer play_arrow
question_answer 115) Which one of the following, is an example of a non-reducing sugar?
A)
Sucrose
done
clear
B)
Lactose
done
clear
C)
Maltose
done
clear
D)
Cellobiose
done
clear
View Answer play_arrow
question_answer 116) The value of Rydberg constant is
A)
\[10,9678\,c{{m}^{-1}}\]
done
clear
B)
\[10,9876\,c{{m}^{-1}}\]
done
clear
C)
\[10,8769\,c{{m}^{-1}}\]
done
clear
D)
\[10,8976\,c{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 117) The wavelength of a spectral line in Lyman series, when electron jumps back from 2nd orbit, is
A)
\[1162\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[1216\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[1362\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[1176\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 118) The number of electrons accommodated in an orbit with principal quantum number 2, is
A)
2
done
clear
B)
6
done
clear
C)
10
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 119) Uranium with a mass number 237 and atomic number 92, changes to a nucleus with atomic number 90 and mass number 233 on emission of
A)
\[\beta -\]particle
done
clear
B)
\[\gamma -\]particle
done
clear
C)
\[\alpha -\]particle
done
clear
D)
positron
done
clear
View Answer play_arrow
question_answer 120) The only, most stable nucleus formed by bombarding, either\[_{13}A{{l}^{27}}\]by neutrons, or\[_{11}N{{a}^{23}}\]by deuterons, is
A)
\[_{15}{{P}^{30}}\]
done
clear
B)
\[_{14}S{{i}^{30}}\]
done
clear
C)
\[_{12}M{{g}^{24}}\]
done
clear
D)
\[_{56}B{{a}^{137}}\]
done
clear
View Answer play_arrow
question_answer 121) The stable electronic configuration of chromium is
A)
\[3{{d}^{6}},\text{ }4{{s}^{1}}\]
done
clear
B)
\[3{{d}^{5}},\text{ }4{{s}^{2}}\]
done
clear
C)
\[3{{d}^{5}},\text{ }4{{s}^{1}}\]
done
clear
D)
\[3{{d}^{6}},\text{ }4{{s}^{0}}\]
done
clear
View Answer play_arrow
question_answer 122) The half-life of\[R{{a}^{226}}\]is 1620 yr, the decay constant (k) is
A)
0.000452
done
clear
B)
0.0004278
done
clear
C)
0.04278
done
clear
D)
0.004278
done
clear
View Answer play_arrow
question_answer 123) According to Lowry and Bronsted, the strength of an acid depends upon
A)
the tendency to gain electrons
done
clear
B)
the tendency to loss protons
done
clear
C)
the tendency to accept protons
done
clear
D)
the tendency to loss electrons
done
clear
View Answer play_arrow
question_answer 124) By applying law of mass action, the equilibrium constant, K for the reaction\[HA+{{H}_{2}}O{{H}_{3}}{{O}^{+}}+\overline{A},\]is given as
A)
\[K=\frac{[HA][{{H}_{2}}O]}{[{{H}_{3}}{{O}^{+}}][\overline{A}]}\]
done
clear
B)
\[K=\frac{[{{H}_{3}}{{O}^{+}}][\overline{A}]}{[HA][{{H}_{2}}O]}\]
done
clear
C)
\[K=\frac{[{{H}_{3}}{{O}^{+}}][{{H}_{2}}O]}{[\overline{A}][HA]}\]
done
clear
D)
\[K=\frac{[HA][\overline{A}]}{[{{H}_{2}}O][{{H}_{3}}{{O}^{+}}]}\]
done
clear
View Answer play_arrow
question_answer 125) The ionisation of strong electrolytes in acetic acid, compared to in water, is
A)
weak, low
done
clear
B)
strong, more
done
clear
C)
medium, the same
done
clear
D)
no ionisation, 100%
done
clear
View Answer play_arrow
question_answer 126) Calculate the pH of a solution in which hydrogen ion concentration is 0.005 g-equi/L?
A)
2.3
done
clear
B)
2.8
done
clear
C)
2.9
done
clear
D)
2.6
done
clear
View Answer play_arrow
question_answer 127) Inversion of cane-sugar in dilute acid is a
A)
bimolecular reaction
done
clear
B)
Pseudo-unimolecular reaction
done
clear
C)
unimolecular reaction
done
clear
D)
trimolecular reaction
done
clear
View Answer play_arrow
question_answer 128) The units of the rate constant of a second order reaction are
A)
\[mo{{l}^{-1}}{{L}^{-1}}{{s}^{-1}}\]
done
clear
B)
\[mo{{l}^{-1}}L\text{ }{{s}^{-1}}\]
done
clear
C)
\[mo{{l}^{-1}}\text{ }L\text{ }s\]
done
clear
D)
\[mol\text{ }{{L}^{-1}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 129) For the first order reaction half-life is 14 s, the time required for the initial concentration to reduce to 1/8 of its value is
A)
\[{{(14)}^{3}}s\]
done
clear
B)
28 s
done
clear
C)
42 s
done
clear
D)
\[{{(14)}^{2}}\text{ }s\]
done
clear
View Answer play_arrow
question_answer 130) One part of solute in one million parts of solvent is expressed as
A)
ppm
done
clear
B)
mg/100 cc
done
clear
C)
g/L
done
clear
D)
g/100 cc
done
clear
View Answer play_arrow
question_answer 131) The molarity of the solution obtained by dissolving 2.5 g of\[NaCl\]in 100 mL of water is
A)
0.00428 moles
done
clear
B)
428 moles
done
clear
C)
0.428 moles
done
clear
D)
0.0428 moles
done
clear
View Answer play_arrow
question_answer 132) The osmotic pressure is expressed in the units of
A)
MeV
done
clear
B)
cal
done
clear
C)
cm/s
done
clear
D)
atm
done
clear
View Answer play_arrow
question_answer 133) Fractional distillation is a process by which the separation of different fractions from mixture of solution is carried by making use of the following property of the fractions
A)
freezing point
done
clear
B)
boiling point
done
clear
C)
melting point
done
clear
D)
solubility
done
clear
View Answer play_arrow
question_answer 134) The quantity of heat measured for a reaction in a bomb calorimeter is equal to
A)
\[\Delta G\]
done
clear
B)
\[\Delta H\]
done
clear
C)
\[p\Delta V\]
done
clear
D)
\[\Delta E\]
done
clear
View Answer play_arrow
question_answer 135) All naturally occurring process, proceed in a direction, which leads to
A)
increase of enthalpy
done
clear
B)
increase of free energy
done
clear
C)
decrease of free energy
done
clear
D)
decrease of entropy
done
clear
View Answer play_arrow
question_answer 136) Heat of combustion of carbon monoxide at constant volume and at\[{{17}^{o}}C\]is\[-67,710\text{ }cal\]. The heat of combustion at constant pressure is
A)
\[-68,000\text{ }cal\]
done
clear
B)
\[-67,800\text{ }cal\]
done
clear
C)
\[-67,050\text{ }cal\]
done
clear
D)
\[+68,500\text{ }cal\]
done
clear
View Answer play_arrow
question_answer 137) The free energy change\[\Delta G=0\]when
A)
the reactants are completely consumed
done
clear
B)
a catalyst is added
done
clear
C)
the system is at equilibrium
done
clear
D)
the reactants are initially mixed
done
clear
View Answer play_arrow
question_answer 138) The units of equivalent conductance, are
A)
\[ohm\text{ }c{{m}^{2}}\text{ }equivalen{{t}^{-1}}\]
done
clear
B)
\[ohm\text{ }c{{m}^{2}}\text{ }equivalent\]
done
clear
C)
\[oh{{m}^{-1}}c{{m}^{2}}equivalen{{t}^{-1}}\]
done
clear
D)
\[mho\text{ }c{{m}^{2}}\text{ }equivalent\]
done
clear
View Answer play_arrow
question_answer 139) The specific conductance (k) of an electrolyte of 0.1 N concentration is related to equivalent conductance by the following formula
A)
\[\Lambda =k\]
done
clear
B)
\[\Lambda =10\,k\]
done
clear
C)
\[\Lambda =100\,k\]
done
clear
D)
\[\Lambda =10,000\,k\]
done
clear
View Answer play_arrow
question_answer 140) The standard electrode potential of hydrogen electrode at 1 M concentration and hydrogen gas at 1 atm pressure is
A)
1 V
done
clear
B)
6 V
done
clear
C)
8 V
done
clear
D)
0 V
done
clear
View Answer play_arrow
question_answer 141) Pure water does not conduct electricity because it is
A)
basic
done
clear
B)
almost not ionized
done
clear
C)
decomposed easily
done
clear
D)
acidic
done
clear
View Answer play_arrow
question_answer 142) The crystalline structure of\[NaCl\]is
A)
hexagonal close packing
done
clear
B)
face centred cubic
done
clear
C)
square planar
done
clear
D)
body centred cubic
done
clear
View Answer play_arrow
question_answer 143) Which one, among the following, is the van der Waals equation, describing the behaviour of one mole of a real gas over wide ranges of temperature and pressure?
A)
\[\left( p+\frac{a}{{{V}^{2}}} \right)(V-b)=RT\]
done
clear
B)
\[\left( p-\frac{a}{{{V}^{2}}} \right)(V-b)=RT\]
done
clear
C)
\[\left( p+\frac{a}{{{V}^{2}}} \right)(V-b)=\frac{R}{T}\]
done
clear
D)
\[\left( p+\frac{a}{{{V}^{2}}} \right)(V+b)=RT\]
done
clear
View Answer play_arrow
question_answer 144) The following is a method to determine the surface tension of liquids
A)
single capillary method
done
clear
B)
refract metric method
done
clear
C)
polarimetric method
done
clear
D)
boiling point method
done
clear
View Answer play_arrow
question_answer 145) The colloidal system of a solid dispersed in liquid medium, is called
A)
aerosol
done
clear
B)
sol
done
clear
C)
gel
done
clear
D)
foam
done
clear
View Answer play_arrow
question_answer 146) Tricarpellary syncarpous superior ovary is seen in
A)
Allium
done
clear
B)
Oenothera
done
clear
C)
Solanum
done
clear
D)
Dolichus
done
clear
View Answer play_arrow
question_answer 147) The edible part in hesperidium fruit is
A)
pericarp
done
clear
B)
mesocarp
done
clear
C)
juicy hair
done
clear
D)
endocarp
done
clear
View Answer play_arrow
question_answer 148) Commercial banana (Musa paradisica) is a
A)
haploid
done
clear
B)
diploid
done
clear
C)
triploid
done
clear
D)
tetraploid
done
clear
View Answer play_arrow
question_answer 149) A technique which involves deliberate manipulation of genes within or between species
A)
Gene therapy
done
clear
B)
Hybridoma technology
done
clear
C)
Tissue culture
done
clear
D)
Genetic engineering
done
clear
View Answer play_arrow
question_answer 150) Genomic DNA library means
A)
a collection of literature about DNA
done
clear
B)
a collection of organisms for extracting DNA
done
clear
C)
packing of donor DNA in a collection of vectors
done
clear
D)
a collection of gene vectors
done
clear
View Answer play_arrow
question_answer 151) Para rubber is obtained from the latex of
A)
Ficus elastic
done
clear
B)
Hevea brasiliensis
done
clear
C)
Carica papaya
done
clear
D)
Musa paradisica
done
clear
View Answer play_arrow
question_answer 152) A good example for organic fertilizer which improves phosphorus uptake is
A)
AM fungi
done
clear
B)
Rhizobium
done
clear
C)
Azospirillum
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 153) In a monohybrid cross involving incomplete dominance the phenotypic ratio equals the genotypic ratio in \[{{F}_{2}}\] generation. The ratio is
A)
3 : 1
done
clear
B)
1 : 2 : 1
done
clear
C)
1 : 1 : 1 : 1
done
clear
D)
9 : 7
done
clear
View Answer play_arrow
question_answer 154) The organism chosen by Mendel to explain the laws of inheritance is
A)
Drosophila melanogaster
done
clear
B)
Antirrhinum majus
done
clear
C)
Pisum sativum
done
clear
D)
Homo sapiens
done
clear
View Answer play_arrow
question_answer 155) When different alleles of the same gene are present in an individual, the individual is a
A)
heterozygous
done
clear
B)
diploid
done
clear
C)
homozygous
done
clear
D)
mosaic
done
clear
View Answer play_arrow
question_answer 156) One of the key factors, which makes the plasmid the vector in genetic engineering is
A)
it is resistant to antibiotics
done
clear
B)
it is resistant to restriction enzymes
done
clear
C)
its ability to carry a foreign gene
done
clear
D)
its ability to cause infection in the host
done
clear
View Answer play_arrow
question_answer 157) The nucleoprotein structures that occur at the ends of the chromosome are
A)
centrosomes
done
clear
B)
telomeres
done
clear
C)
centromeres
done
clear
D)
satellites
done
clear
View Answer play_arrow
question_answer 158) During the replication of DNA, the synthesis of DNA on lagging strand takes place in segments. These segments are called
A)
double helix segments
done
clear
B)
satellite segments
done
clear
C)
Komberg segments
done
clear
D)
Okazaki segments
done
clear
View Answer play_arrow
question_answer 159) The codon AUG is
A)
ochre
done
clear
B)
amber
done
clear
C)
initiation codon
done
clear
D)
termination codon
done
clear
View Answer play_arrow
question_answer 160) RNA polymerase contains multiple polypeptide units. For initiating RNA synthesis it requires
A)
\[\sigma \]-subunit
done
clear
B)
\[\beta \]-subunit
done
clear
C)
\[\text{ }\!\!\rho\!\!\text{ -}\]factor
done
clear
D)
spliceosome
done
clear
View Answer play_arrow
question_answer 161) In multicellular organisms the 70S ribosomes a. jund in the following parts of the cells
A)
lysosomes
done
clear
B)
mitochondria
done
clear
C)
nucleus
done
clear
D)
endoplasmic reticulum
done
clear
View Answer play_arrow
question_answer 162) The cavities of alveoli of lungs are lined by
A)
cuboidal epithelium
done
clear
B)
columnar epithelium
done
clear
C)
stratified cuboidal epithelium
done
clear
D)
squamous epithelium
done
clear
View Answer play_arrow
question_answer 163) The deficiency of a vitamin which causes keratomalacia is
A)
vitamin-K
done
clear
B)
vitamin-D
done
clear
C)
vitamin-A
done
clear
D)
vitamin-E
done
clear
View Answer play_arrow
question_answer 164) The condition in which the potassium levels are increased is known as
A)
hypercholesteremia
done
clear
B)
hyperkalemia
done
clear
C)
osteomalacia
done
clear
D)
hyperexcitability
done
clear
View Answer play_arrow
question_answer 165) Hambergers phenomenon explains
A)
formation of \[HC{{O}_{3}}\]
done
clear
B)
chloride shift
done
clear
C)
oxygen saturation of haemoglobin
done
clear
D)
breathing mechanism
done
clear
View Answer play_arrow
question_answer 166) Open type of vascular system is predominant in
A)
fishes
done
clear
B)
amphibians
done
clear
C)
arthropods
done
clear
D)
earthworm
done
clear
View Answer play_arrow
question_answer 167) The hormone which helps in the reabsorption of water in kidney tubules is
A)
cholecystokinin
done
clear
B)
angiotensin
done
clear
C)
anti diuretic hormone
done
clear
D)
pancreozymin
done
clear
View Answer play_arrow
question_answer 168) The net filtration pressure in the glomerulus of the kidney is
A)
70 mm Hg
done
clear
B)
35 mm Hg
done
clear
C)
25 mm Hg
done
clear
D)
10 mm Hg
done
clear
View Answer play_arrow
question_answer 169) Prostate gland is a
A)
digestive gland
done
clear
B)
sperm producing gland
done
clear
C)
semen secreting accessory gland of male
done
clear
D)
hormone producing gland of the ovary
done
clear
View Answer play_arrow
question_answer 170) The hormone testosterone is secreted in testis by
A)
spermatogonia
done
clear
B)
Leydig cells
done
clear
C)
Sertoli cells
done
clear
D)
epididymis
done
clear
View Answer play_arrow
question_answer 171) The one way or unidirectional transmission of nerve impulse in nerve cells is due to the presence of
A)
synapses
done
clear
B)
myelin sheath
done
clear
C)
membrane polarity
done
clear
D)
intemeurons
done
clear
View Answer play_arrow
question_answer 172) Which one of the following is the hormone of adrenal medulla?
A)
Prolactin
done
clear
B)
ACTH
done
clear
C)
Corticosterone
done
clear
D)
Epinephrine
done
clear
View Answer play_arrow
question_answer 173) The cell division that takes place in a zygote is known as
A)
meiosis
done
clear
B)
mitosis
done
clear
C)
cleavage
done
clear
D)
differentiation
done
clear
View Answer play_arrow
question_answer 174) The excretory cells that are found in Platyhelminthes are
A)
protonephridia
done
clear
B)
flame cells
done
clear
C)
solenocytes
done
clear
D)
chlorogogen cells
done
clear
View Answer play_arrow
question_answer 175) Wuchereria bancrofti is a common filarial worm. It belongs to phylum
A)
Platyhelminthes
done
clear
B)
Nemathelminthes
done
clear
C)
Annelida
done
clear
D)
Coelenterata
done
clear
View Answer play_arrow
question_answer 176) Exoskeleton of the following consists of a chitinous cuticle
A)
Annelida
done
clear
B)
Porifera
done
clear
C)
Arthropoda
done
clear
D)
Echinodermata
done
clear
View Answer play_arrow
question_answer 177) Chitin is a
A)
lipid
done
clear
B)
protein
done
clear
C)
polysaccharide
done
clear
D)
sphingomyelin
done
clear
View Answer play_arrow
question_answer 178) One of the following has a biradial symmetry
A)
Paramecium
done
clear
B)
jellyfish
done
clear
C)
cockroach
done
clear
D)
sea anemone
done
clear
View Answer play_arrow
question_answer 179) Which of the following animals has a true coelom?
A)
Ascaris
done
clear
B)
Pheretima
done
clear
C)
Sycon
done
clear
D)
Taenia solium
done
clear
View Answer play_arrow
question_answer 180) Survival of the fittest is the basic principle of a competition. Its importance in organic evolution was explained by
A)
Lamarck
done
clear
B)
de Vries
done
clear
C)
Darwin
done
clear
D)
Mendel
done
clear
View Answer play_arrow
question_answer 181) Vestigial organs present in an adult individual are examples of basis of evidences of evolution.
A)
morphological
done
clear
B)
paleontological
done
clear
C)
embryological
done
clear
D)
anatomical
done
clear
View Answer play_arrow
question_answer 182) The earliest members of Hominids, which evolved more four million year ago belong to
A)
Homo erectus
done
clear
B)
Cro-magnon man
done
clear
C)
Australopithecus
done
clear
D)
Neanderthal man
done
clear
View Answer play_arrow
question_answer 183) An example of reproductive isolation is
A)
Archeopteryx
done
clear
B)
dinosaurs
done
clear
C)
mule
done
clear
D)
Bonelia
done
clear
View Answer play_arrow
question_answer 184) The theory of random genetic drift was proposed by
A)
Hardy-Weinberg
done
clear
B)
R A Fisher
done
clear
C)
Sewall Wright
done
clear
D)
May r
done
clear
View Answer play_arrow
question_answer 185) One of the protozoan disease is
A)
polio
done
clear
B)
AIDS
done
clear
C)
taeniasis
done
clear
D)
malaria
done
clear
View Answer play_arrow
question_answer 186) Insulin deficiency is a causative factor for
A)
taeniasis
done
clear
B)
diabetes
done
clear
C)
tuberculosis
done
clear
D)
gonorrhea
done
clear
View Answer play_arrow
question_answer 187) The test which is misused for identification of an unborn baby is
A)
clotting test
done
clear
B)
amniocentesis
done
clear
C)
erythroblastosis
done
clear
D)
angiogram
done
clear
View Answer play_arrow
question_answer 188) One of the following blood group person cannot donate blood to others
A)
AB blood group
done
clear
B)
O blood group
done
clear
C)
A blood group
done
clear
D)
B blood group
done
clear
View Answer play_arrow
question_answer 189) The possibility of erythroblastosis foetalis occurring during the second pregnancy is when
A)
the baby is \[R{{h}^{+}}\] and mother \[R{{h}^{-}}\]
done
clear
B)
the baby is \[R{{h}^{+}}\]and mother \[R{{h}^{+}}\]
done
clear
C)
the baby is \[R{{h}^{-}}\]and mother \[R{{h}^{-}}\]
done
clear
D)
the baby is \[R{{h}^{-}}\]and mother \[R{{h}^{+}}\]
done
clear
View Answer play_arrow
question_answer 190) One of the inflammatory reactions induced by histamine is
A)
vasoconstriction of blood vessels
done
clear
B)
vasodilation of peripheral blood vessels
done
clear
C)
increased vascular permeability
done
clear
D)
accelerated blood clotting
done
clear
View Answer play_arrow
question_answer 191) Movement of cytoplasm around the vacuole in the cell is called as
A)
circulation
done
clear
B)
rotation
done
clear
C)
somersault
done
clear
D)
regulation
done
clear
View Answer play_arrow
question_answer 192) Soft-rot disease of sweet potato is caused by
A)
Rhizopus stolonifer
done
clear
B)
Rhizopus sexualis
done
clear
C)
Chlamydomonas nivalis
done
clear
D)
Chlamydomonas coccifera
done
clear
View Answer play_arrow
question_answer 193) In Funaria antheridial branch is called
A)
male flower
done
clear
B)
female head
done
clear
C)
male cone
done
clear
D)
female cone
done
clear
View Answer play_arrow
question_answer 194) Pollen grains in Pinus are
A)
monosaccate
done
clear
B)
bisaccate
done
clear
C)
trisaccate
done
clear
D)
nonsaccate
done
clear
View Answer play_arrow
question_answer 195) Pits are formed on the cell wall is due to lack of
A)
cell plate
done
clear
B)
primary wall material
done
clear
C)
secondary wall material
done
clear
D)
middle lamellum
done
clear
View Answer play_arrow
question_answer 196) Coupling factor F is found in
A)
stroma
done
clear
B)
matrix
done
clear
C)
thylakoids
done
clear
D)
ribosomes
done
clear
View Answer play_arrow
question_answer 197) The longest chromosome is seen in
A)
Allium
done
clear
B)
Lilium
done
clear
C)
Trillium
done
clear
D)
Zea mays
done
clear
View Answer play_arrow
question_answer 198) Nucleotide consists of
A)
phosphate only
done
clear
B)
phosphate and sugar only
done
clear
C)
phosphate, sugar and nitrogen base
done
clear
D)
phosphate and nitrogen base only
done
clear
View Answer play_arrow
question_answer 199) Ribosomes are attached to endoplasmic reticulum through
A)
ribophorin
done
clear
B)
magnesium
done
clear
C)
peptidyl transferase
done
clear
D)
tRNA
done
clear
View Answer play_arrow
question_answer 200) Root modification means
A)
permanent internal changes in the roots
done
clear
B)
temporary internal changes in the roots
done
clear
C)
permanent structural changes in the roots
done
clear
D)
temporary structural changes in the roots
done
clear
View Answer play_arrow
question_answer 201) Scaly bulb stem modification is seen in
A)
Allium
done
clear
B)
Lilium
done
clear
C)
Scilla
done
clear
D)
Ginger
done
clear
View Answer play_arrow
question_answer 202) Tendrils in Passiflora develops from
A)
apical roots
done
clear
B)
apices of roots
done
clear
C)
axillary buds
done
clear
D)
terminal buds
done
clear
View Answer play_arrow
question_answer 203) The secondary meristem initiates
A)
basal growth
done
clear
B)
transverse growth
done
clear
C)
radial growth
done
clear
D)
vertical growth
done
clear
View Answer play_arrow
question_answer 204) Lamellar collenchyma is seen in the stem of
A)
Cucurbita
done
clear
B)
Leucas
done
clear
C)
Sambucus
done
clear
D)
Monstera
done
clear
View Answer play_arrow
question_answer 205) Vessels are absent in this angiosperm
A)
Mangifera
done
clear
B)
Magnolia
done
clear
C)
Dillenia
done
clear
D)
Drimys
done
clear
View Answer play_arrow
question_answer 206) The epidermal trichomes help in
A)
transpiration and exchange of gases
done
clear
B)
protection from desiccation
done
clear
C)
protection and reduction of transpiration
done
clear
View Answer play_arrow
question_answer 207) Alburnum is also called as
A)
autumn wood
done
clear
B)
heart wood
done
clear
C)
sap wood
done
clear
D)
spring wood
done
clear
View Answer play_arrow
question_answer 208) Periderm is produced from
A)
cork cambium
done
clear
B)
pro-cambium
done
clear
C)
secondary cortex
done
clear
D)
vascular cambium
done
clear
View Answer play_arrow
question_answer 209) Simple sieve plate with single perforation is found in
A)
Cucurbita
done
clear
B)
Prunus
done
clear
C)
Pyrus
done
clear
D)
Vitis
done
clear
View Answer play_arrow
question_answer 210) This is a specialized tissue found in the mesophyll of Cycas and Pinus leaves
A)
spongy tissue
done
clear
B)
palisade tissue
done
clear
C)
conjunctive tissue
done
clear
D)
transfusion tissue
done
clear
View Answer play_arrow
question_answer 211) Bicollateral vascular bundles are found in the members of this family
A)
Malvaceae
done
clear
B)
Fabaceae
done
clear
C)
Caesalpiniaceae
done
clear
D)
Cucurbitaceae
done
clear
View Answer play_arrow
question_answer 212) Element which regulates stomatal movement
A)
potassium
done
clear
B)
sodium
done
clear
C)
sulphur
done
clear
D)
phosphorus
done
clear
View Answer play_arrow
question_answer 213) Mottled chlorosis on the leaves occurs due to the deficiency of
A)
nitrogen
done
clear
B)
phosphorus
done
clear
C)
potassium
done
clear
D)
sulphur
done
clear
View Answer play_arrow
question_answer 214) The type of \[C{{O}_{2}}\]fixation seen in many succulent plant species is
A)
\[{{\text{C}}_{\text{4}}}\text{-}\]pathway
done
clear
B)
\[{{\text{C}}_{\text{2}}}\text{-}\]pathway
done
clear
C)
CAM-pathway
done
clear
D)
\[{{\text{C}}_{3}}\text{-}\]pathway
done
clear
View Answer play_arrow
question_answer 215) In the production of ethanol, pyruvic acid is first converted to acetaldehyde by this enzyme
A)
alcohol dehydrogenase
done
clear
B)
alcohol oxidase
done
clear
C)
pyruvate dehydrogenase
done
clear
D)
pyruvate decarboxylase
done
clear
View Answer play_arrow
question_answer 216) Beta vulgaris is a
A)
short day plant
done
clear
B)
long day plant
done
clear
C)
day neutral plant
done
clear
D)
intermediate plant
done
clear
View Answer play_arrow
question_answer 217) It will be useful in artificial ripening of fruits
A)
2, 4-D
done
clear
B)
ethylene
done
clear
C)
cytokinin
done
clear
D)
gibberellins
done
clear
View Answer play_arrow
question_answer 218) Rapid and dramatic increase in shoot length is called
A)
triple response growth
done
clear
B)
bolting
done
clear
C)
scarification
done
clear
D)
night break effect
done
clear
View Answer play_arrow
question_answer 219) Enzyme nitrogenase is responsible for
A)
nitrification
done
clear
B)
nitrogen fixation
done
clear
C)
nitrite reduction
done
clear
D)
nitrate reduction
done
clear
View Answer play_arrow
question_answer 220) Scorpioid cyme is seen in
A)
Hamelia
done
clear
B)
Heliotropium
done
clear
C)
Clerodendron
done
clear
D)
Nerium
done
clear
View Answer play_arrow