General
methods of preparation of Alkyl Halides
(1)
From alkanes
(i)
By halogenation: \[\underset{\text{Ethane}}{\mathop{{{C}_{2}}{{H}_{6}}}}\,\]
(Excess) +\[C{{l}_{2}}\xrightarrow{hv}\underset{\text{Ethyl}\,\text{chloride
(Major}\,\text{product)}}{\mathop{{{C}_{2}}{{H}_{5}}Cl}}\,+HCl\]
\[\underset{\text{Propane}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{3}}}}\,\overset{C{{l}_{2}}}{\mathop{\xrightarrow[UV\,light]{}}}\,\underset{1-\text{Chloropropane
(45 }\!\!%\!\!\text{
)}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Cl}}\,\,\underset{\text{2-
Chloropropane (55 }\!\!%\!\!\text{ )}}{\mathop{\underset{\,Cl}{\mathop{\underset{|}{\mathop{+\
C{{H}_{3}}CHC{{H}_{3}}}}\,}}\,}}\,\]
This
reaction proceed through free radical mechanism.
Note: r
Order of reactivity of \[{{X}_{2}}\] for a given alkane is, \[{{F}_{2}}>C{{l}_{2}}>B{{r}_{2}}>{{I}_{2}}\].
r The
reactivity of the alkanes follows the order: \[3{}^\circ alkane\text{
}>~2{}^\circ alkane\text{ }>~1{}^\circ alkane\].
(ii)
With sulphuryl chloride: \[R-H+S{{O}_{2}}C{{l}_{2}}\overset{hv}{\mathop{\xrightarrow[Organic\,peroxide{{(R'C{{O}_{2}})}_{2}}]{}}}\,R-Cl+S{{O}_{2}}+HCl\]
Note: r In
presence of light and trace of an organic peroxide the reaction is fast.
(2)
From alkenes (Hydrohalogenation)
\[\underset{\text{But}-\text{2}-\text{ene}}{\mathop{C{{H}_{3}}-CH=CH-C{{H}_{3}}+HBr}}\,\xrightarrow{{}}\underset{\text{2-Bromobutane}}{\mathop{C{{H}_{3}}C{{H}_{2}}-\underset{Br\,\,\,\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,H-}}\,C{{H}_{3}}}}\,\xrightarrow{{}}\]Electrophillic
addition.
Note: r
Addition of HBr to alkene in the presence of organic peroxide take place due to
peroxide effect or Kharasch's effect.
r This addition take place by two
mechanism,
Peroxide
initiates free radical mechanism.
Markownikoff?s
addition by electrophillic
more...
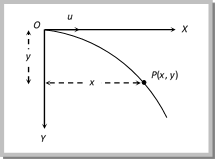 The vertical displacement y is governed by \[y=\frac{1}{2}g{{t}^{2}}\] ?. (ii)
(since initial vertical velocity is zero)
By substituting the value of t in equation (ii) \[y=\frac{1}{2}\frac{g\,{{x}^{2}}}{{{u}^{2}}}\]
Sample problems based on trajectory
Problem 66. An aeroplane is flying at more...
The vertical displacement y is governed by \[y=\frac{1}{2}g{{t}^{2}}\] ?. (ii)
(since initial vertical velocity is zero)
By substituting the value of t in equation (ii) \[y=\frac{1}{2}\frac{g\,{{x}^{2}}}{{{u}^{2}}}\]
Sample problems based on trajectory
Problem 66. An aeroplane is flying at more...