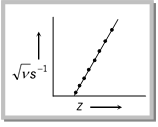
 Z= atomic number of the metal
\[a\And b\] are constant.
(iii) Atomic number = Number of positive charge on nucleus = Number of protons in nucleus = Number of electrons in nutral atom.
(iv) Two different elements can never have identical atomic number.
(2) Mass number
(i) more...
Z= atomic number of the metal
\[a\And b\] are constant.
(iii) Atomic number = Number of positive charge on nucleus = Number of protons in nucleus = Number of electrons in nutral atom.
(iv) Two different elements can never have identical atomic number.
(2) Mass number
(i) more...
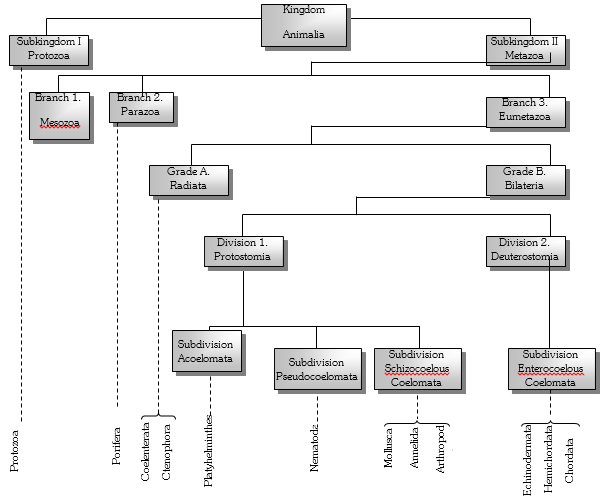 Subkingdom 1. Protozoa : It includes microscopic, unicellular animals. It contains a single Phylum called protozoa. more...
Subkingdom 1. Protozoa : It includes microscopic, unicellular animals. It contains a single Phylum called protozoa. more...
You need to login to perform this action.
You will be redirected in
3 sec
