| Disease | Causative Bacterium |
|
(1)
Cholera
(2)
Pneumonia
(3)
Typhoid
(4)
Tetanus
(5)
Diphtheria
Diseases caused by Viruses
(a) Important Diseases
caused by Viruses: The human diseases caused by viruses include influenza,
chickenpox, smallpox measles, rabies, mumps, polio, trachoma, hepatitis and
AIDS.
(1) Influenza: Influenza,
commonly called flu, is a highly infectious disease, which has still not been
conquered. It is caused by many kinds of viruses, such as myxovirus. The latter
affect the mucous membrane of nose, throat and upper respiratory tract. The
common symptoms are discharge from the nose, sneezing, fever, body aches,
coughing and general weakness. The infection spreads by discharges from the
nose and throat. The incubation period is just from 24-72 hours. Influenza
generally lasts for 4 or 5 days. Rest quickens the recovery. If neglected,
complications like pneumonia, bronchitis and ear infection may develop. There
is no vaccine for influenza.
Influenza tends
to occur in epidemic more...
Free Energy
and Work Function
Gibb's
free energy (G) is a state function and is a measure of maximum
work done or useful work done from a reversible reaction at constant
temperature and pressure.
(1)
Characteristics of free energy
(i) The
free energy of a system is the enthalpy of the system minus the product of
absolute temperature and entropy i.e., \[G=H-TS\]
(ii)
Like other state functions E, H and S, it is also expressed as \[\Delta
G\]. Also \[\Delta G=\Delta H-T\Delta {{S}_{system}}\]where \[\Delta S\] is
entropy change for system only. This is Gibb's Helmholtz equation.
(iii)
At equilibrium \[\Delta G=0\]
(iv)
For a spontaneous process decrease in free energy is noticed i.e., \[\Delta
G=-ve\].
(v) At
absolute zero, \[T\Delta S\]is zero. Therefore if \[\Delta G\]is ? ve, \[\Delta
H\]should be ? ve or only exothermic reactions proceed spontaneously at
absolute more...
Bond Energy
or Bond Enthalpies
When a bond is formed
between atoms, energy is released. Obviously same amount of energy will be
required to break the bond. The energy required to break the bond is termed bond
dissociation energy. The more precise definition is,
?The amount
of energy required to break one mole of bond of a particular type between the
atoms in the gaseous state, i.e., to separate the atoms in the gaseous state
under 1 atmospheric pressure and the specified temperature is called bond
dissociation energy.?
For example, \[H-H(g)\to
2H(g);\] \[\Delta H=+\,433\,kJ\,mo{{l}^{-1}}\]
\[Cl-Cl(g)\to
2Cl\,(g);\] \[\Delta H=+\,242.5\,kJ\,mo{{l}^{-1}}\]
\[H-Cl(g)\,\to
H(g)+Cl(g);\]\[\Delta H=+\,431\,kJ\,mo{{l}^{-1}}\]
\[I-I(g)\to
2I(g);\] \[\Delta H=+\,15.1\,kJ\,mo{{l}^{-1}}\]
\[H-I(g)\to
H(g)+I(g);\] \[\Delta H=+\,299\,kJ\,mo{{l}^{-1}}\]
The bond dissociation
energy of a diatomic molecule is also called bond energy.
However, the bond dissociation energy depends upon the nature of bond and also
the molecule in which more...
Heat of Reaction
Heat of reaction is
defined as the amount of heat evolved or absorbed when quantities of the
substances indicated by the chemical equation have completely reacted. The heat
of reaction (or enthalpy of reaction) is actually the difference between the
enthalpies of the products and the reactants when the quantities of the
reactants indicated by the chemical equation have completely reacted.
Mathematically,
Enthalpy
of reaction (heat of reaction) \[=\Delta H=\Sigma {{H}_{P}}-\Sigma {{H}_{R}}\]
(1) Factors which
influence the heat of reaction :
There are a number of factors which affect the magnitude of heat of reaction.
(i) Physical state
of reactants and products :
Heat energy is involved for changing the physical state of a chemical
substance. more...
Second Law
of Thermodynamics
All the limitations of
the first law of thermodynamics can be remove by the second law of
thermodynamics. This law is generalisation of certain experiences about heat
engines and refrigerators. It has been stated in a number of ways, but all the
statements are logically equivalent to one another.
(1)
Statements of the law
(i) Kelvin
statement : ?It is impossible to derive a continuous supply of work
by cooling a body to a temperature lower than that of the coldest of its
surroundings.?
(ii) Clausius
statement : ?It
is impossible for a self acting machine, unaided by any external agency, to
convert heat from one body to another at a higher temperature or Heat cannot itself pass from a
colder body to more...
Basic Terms of Thermodynamics
Thermodynamics
Thermodynamics (thermo means heat and dynamics means motion) is the branch of science which deals with the study of different forms of energy and the quantitative relationships between them.
The complete study of thermodynamics is based upon three generalizations celled first, second and third laws of thermodynamics. These laws have been arrived purely on the basis of human experience and there is no theoretical proof for any of these laws.
(1) System, surroundings and Boundary : A specified part of the universe which is under observation is called the system and the remaining portion of the universe which is not a part of the system is called the surroundings.
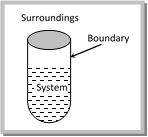 The system and the surroundings are separated by real or imaginary boundaries. The boundary more...
The system and the surroundings are separated by real or imaginary boundaries. The boundary more...
Articles CategoriesArchive
Trending Articles
You need to login to perform this action. |