
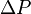 is the hydrostatic pressure (given in pascals in the SI system), or the difference in pressure at two points within a fluid column, due to the weight of the fluid;
ρ is the fluid density (in kilograms per cubic meter more...
is the hydrostatic pressure (given in pascals in the SI system), or the difference in pressure at two points within a fluid column, due to the weight of the fluid;
ρ is the fluid density (in kilograms per cubic meter more...
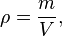 where ρ is the density, m is the mass, and V is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume,although this is scientifically inaccurate – this quantity is more properly called specific weight.
Visit http://www.studyadda.com/videos/ix-class-physics-lectures/floatation/density/1744
All the video lectures at StudyAdda are exclusively developed and taught by Mr. Lalit Sardana(IIT-JEE,AIR 243) and Mrs. Shweta Sardana(AIPMT Trainer, MSc., MPhil (Gold Medalist)), Sardana Tutorials).
where ρ is the density, m is the mass, and V is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume,although this is scientifically inaccurate – this quantity is more properly called specific weight.
Visit http://www.studyadda.com/videos/ix-class-physics-lectures/floatation/density/1744
All the video lectures at StudyAdda are exclusively developed and taught by Mr. Lalit Sardana(IIT-JEE,AIR 243) and Mrs. Shweta Sardana(AIPMT Trainer, MSc., MPhil (Gold Medalist)), Sardana Tutorials).
You need to login to perform this action.
You will be redirected in
3 sec
