-
question_answer1) In producing chlorine through electrolysis 100 W power at 125 V is being consumed. How much chlorine per min is liberated? ECE of chlorine is \[0.367\times {{10}^{-6}}\,kg/C\]:
A)
17.6 mg
done
clear
B)
21.3 mg
done
clear
C)
24.3 mg
done
clear
D)
13.6 mg
done
clear
View Answer play_arrow
-
question_answer2)
In die circuit shown, if a conducting wire is connected between points A and B, the current in this wire will: 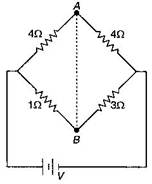
A)
flow from A to B
done
clear
B)
flow in the direction which will be decided by the value of V
done
clear
C)
be zero
done
clear
D)
flow from B to A
done
clear
View Answer play_arrow
-
question_answer3) A rectangular block of mass m and area of cross-section A floats in a liquid of density \[\rho \]. If it is given a small vertical displacement from equilibrium it undergoes oscillation with a time period T. Then:
A)
\[T\propto \,\,\sqrt{\rho }\]
done
clear
B)
\[T\propto \,\,\frac{1}{\sqrt{A}}\]
done
clear
C)
\[T\propto \,\,\frac{1}{\rho }\]
done
clear
D)
\[T\propto \,\,\frac{1}{\sqrt{m}}\]
done
clear
View Answer play_arrow
-
question_answer4) A Carnot engine whose sink is at 300 K has an efficiency of 40%. By how much should the temperature of source be increased so as to increase its efficiency by 50% of original efficiency?
A)
275 K
done
clear
B)
325 K
done
clear
C)
250 K
done
clear
D)
380 K
done
clear
View Answer play_arrow
-
question_answer5) When a charged particle moving with velocity \[\vec{v}\] is subjected to a magnetic field of induction \[\vec{B}\], the force on it is non-zero. This implies that:
A)
angle between \[\vec{v}\] and \[\vec{B}\] is necessarily 90°
done
clear
B)
angle between \[\vec{v}\] and \[\vec{B}\] can have any value other than 90°
done
clear
C)
angle between \[\vec{v}\] and \[\vec{B}\] can have any value other than zero and 180°
done
clear
D)
angle between \[\vec{v}\] and \[\vec{B}\] is either zero or \[{{180}^{o}}\]
done
clear
View Answer play_arrow
-
question_answer6) Two cells, having the same emf, are connected in series through an external resistance R. Cells have internal resistances \[{{r}_{1}}\] and \[{{r}_{2}}({{r}_{1}}>{{r}_{2}})\] respectively. When the circuit is closed, the potential difference across the first cell is zero. The value of R is:
A)
\[{{r}_{1}}-{{r}_{2}}\]
done
clear
B)
\[\frac{{{r}_{1}}+{{r}_{2}}}{2}\]
done
clear
C)
\[\frac{{{r}_{1}}-{{r}_{2}}}{2}\]
done
clear
D)
\[{{r}_{1}}+{{r}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer7) A black body at \[{{1227}^{o}}C\] emits radiations with maximum intensity at a wavelength of \[5000\,\overset{o}{\mathop{A}}\,\]. If the temperature of the body is increased by \[{{1000}^{o}}C\], the maximum intensity will be observed at:
A)
\[4000\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[5000\,\overset{o}{\mathop{A}}\,\]
done
clear
C)
\[6000\,\overset{o}{\mathop{A}}\,\]
done
clear
D)
\[3000\,\overset{o}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
-
question_answer8) Two circular coils 1 and 2 are made from the same wire but the radius of the 1st coil is twice that of die 2nd coil. What is the ratio of potential difference applied across them so that die magnetic field at their centres is the same?
A)
3
done
clear
B)
4
done
clear
C)
6
done
clear
D)
2
done
clear
View Answer play_arrow
-
question_answer9) A transistor-oscillator using a resonant circuit with an inductor L (of negligible resistance) and a capacitor C in series produce oscillations of frequency f. If L is doubled and C is changed to 4C, the frequency will be:
A)
\[f/4\]
done
clear
B)
\[8f\]
done
clear
C)
\[f/2\sqrt{2}\]
done
clear
D)
\[f/2\]
done
clear
View Answer play_arrow
-
question_answer10) The binding energy of deuteron is 2.2 MeV and that of \[_{2}^{4}He\] is 28 MeV. If two deuterons are fused to form one \[_{2}^{4}He\] then the energy released is:
A)
25.8 MeV
done
clear
B)
23.6 MeV
done
clear
C)
19.2 MeV
done
clear
D)
30.2 MeV
done
clear
View Answer play_arrow
-
question_answer11) In a radioactive material die activity at time \[{{t}_{1}}\] is \[{{R}_{1}}\] and at a later time \[{{t}_{2}}\], it is \[{{R}_{2}}\]. If the dacay constant of the material is \[\lambda \], then:
A)
\[{{R}_{1}}={{R}_{2}}\,{{e}^{-\lambda ({{t}_{1}}-{{t}_{2}})}}\]
done
clear
B)
\[{{R}_{1}}={{R}_{2}}\,{{e}^{\lambda ({{t}_{1}}-{{t}_{2}})}}\]
done
clear
C)
\[{{R}_{1}}={{R}_{2}}\,({{t}_{2}}/{{t}_{1}})\]
done
clear
D)
\[{{R}_{1}}={{R}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer12) Ionization potential of hydrogen atom is 13.6 eV. Hydrogen atoms in the ground state are excited by monochromatic radiation of photon energy 12.1 eV. According to Bohr's theory, the spectral lines emitted by hydrogen will be:
A)
two
done
clear
B)
three
done
clear
C)
four
done
clear
D)
one
done
clear
View Answer play_arrow
-
question_answer13) The potential energy of a long spring when stretched by 2 cm is U. If the spring is stretched by 8 cm the potential energy stored in it is:
A)
4U
done
clear
B)
8U
done
clear
C)
16U
done
clear
D)
U/4
done
clear
View Answer play_arrow
-
question_answer14) For angles of projection of a projectile at angles \[({{45}^{o}}-\theta )\] and \[({{45}^{o}}+\theta )\] the horizontal ranges described by the projectile are in the ratio of:
A)
1 : 1
done
clear
B)
2 : 3
done
clear
C)
1 : 2
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
-
question_answer15) A body of mass 3 kg is under a constant force which causes a displacement s in metres in it, given by the relation \[s=\frac{1}{3}\,{{t}^{2}},\] where t is in s. Work done by the force in 2 s is:
A)
\[\frac{5}{19}\,J\]
done
clear
B)
\[\frac{3}{18}\,J\]
done
clear
C)
\[\frac{8}{3}\,J\]
done
clear
D)
\[\frac{19}{5}\,J\]
done
clear
View Answer play_arrow
-
question_answer16) A particle moves along a straight line OX. At a time t (in seconds) the distance x (in metres) of the particle from O is given by \[x=40+12t-{{t}^{3}}\] How long would the particle travel before coming to rest?
A)
24 m
done
clear
B)
40 m
done
clear
C)
56 m
done
clear
D)
16 m
done
clear
View Answer play_arrow
-
question_answer17) The velocity v of a particle at time t is given by \[v=at+\frac{b}{t+c},\] where a, b and c are constants. The dimensions of a, b and c are respectively:
A)
\[[L{{T}^{-2}}],\,[L]\] and \[[T]\]
done
clear
B)
\[[{{L}^{2}}],\,[T]\] and \[[L{{T}^{2}}]\]
done
clear
C)
\[[L{{T}^{2}}],\,[LT]\] and \[[L]\]
done
clear
D)
\[[L],\,[LT]\] and \[[{{T}^{2}}]\]
done
clear
View Answer play_arrow
-
question_answer18) A microscope is focussed on a mark on a piece of paper and then a slab of glass of thickness 3 cm and refractive index 1.5 is placed over the mark. How should the microscope be moved to get the mark in focus again?
A)
1 cm upward
done
clear
B)
4.5 cm downward
done
clear
C)
1 cm downward
done
clear
D)
2 cm upward
done
clear
View Answer play_arrow
-
question_answer19) 300 J of work is done in sliding a 2 kg block up an inclined plane of height 10 m. Taking \[g=10\,m/{{s}^{2}},\] work done against friction is:
A)
200 J
done
clear
B)
100 J
done
clear
C)
zero
done
clear
D)
1000 J
done
clear
View Answer play_arrow
-
question_answer20) A transistor is operated in common emitter configuration at constant collector voltage \[{{V}_{c}}=1.5\,\,V\] such that a change in the base current from 100\[\mu \]A to 150\[\mu \]A produces a change in the collector current from 5 mA to 10 mA. The current gain \[(\beta )\] is:
A)
67
done
clear
B)
75
done
clear
C)
100
done
clear
D)
50
done
clear
View Answer play_arrow
-
question_answer21) A forward biased diode is:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer22) A photo-cell employs photoelectric effect to convert:
A)
change in the frequency of light into a change in electric voltage
done
clear
B)
change in the intensity of illumination into change in photoelectric current
done
clear
C)
change in the intensity of illumination into a change in the work function of the photocathode
done
clear
D)
change in the frequency of light into a change in the electric current
done
clear
View Answer play_arrow
-
question_answer23) The core of a transformer is laminated because:
A)
energy losses due to eddy currents may be minimized.
done
clear
B)
the weight of the transformer may be reduced
done
clear
C)
rusting of the core may be prevented
done
clear
D)
ratio of voltage in primary and secondary may be increased
done
clear
View Answer play_arrow
-
question_answer24) Two coils of self-inductances 2 mH and 8 mH are placed so close together that the effective flux in one coil is completely linked with the other. The mutual inductance between these coils is:
A)
10 mH
done
clear
B)
6 mH
done
clear
C)
4 mH
done
clear
D)
16 mH
done
clear
View Answer play_arrow
-
question_answer25) In a discharge tube ionization of enclosed gas is produced due to collisions between:
A)
positive ions and neutral atoms/molecules
done
clear
B)
negative electrons and neutral atoms/molecules
done
clear
C)
photons and neutral atoms/molecules
done
clear
D)
neutral gas atoms/molecules
done
clear
View Answer play_arrow
-
question_answer26) When photons of energy hv fall on an aluminium plate (of work function \[{{E}_{0}}\]), photoelectrons of maximum kinetic energy K are ejected. If the frequency of the radiation is doubled, the maximum kinetic energy of die ejected photoelectrons will be:
A)
\[K+{{E}_{0}}\]
done
clear
B)
\[2K\]
done
clear
C)
\[K\]
done
clear
D)
\[K+hv\]
done
clear
View Answer play_arrow
-
question_answer27)
The following figure shows a logic gate circuit with two inputs A and B and the output C. The voltage waveforms of A, B and C are as shown below: 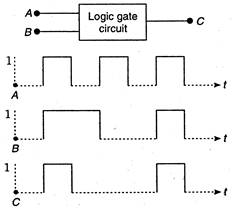 The logic circuit gate is:
The logic circuit gate is:
A)
AND gate
done
clear
B)
NAND gate
done
clear
C)
NOR gate
done
clear
D)
OR gate
done
clear
View Answer play_arrow
-
question_answer28) A coil of inductive reactance \[31\,\,\Omega \]. has a resistance of \[8\,\,\Omega \]. It is placed in series with a condenser of capacitative reactance \[25\,\,\Omega \]. The combination is connected to an a.c. source of 110 V. The power factor of the circuit is:
A)
0.56
done
clear
B)
0.64
done
clear
C)
0.80
done
clear
D)
0.33
done
clear
View Answer play_arrow
-
question_answer29)
A 0.5 kg ball moving with a speed of 12 m/s strikes a hard wall at an angle of 30° with the wall. It is reflected with the same speed and at the same angle. If the ball is in contact with the wall for 0.25 s, the average force acting on the wall is: 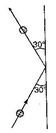
A)
48 N
done
clear
B)
24 N
done
clear
C)
12 N
done
clear
D)
96 N
done
clear
View Answer play_arrow
-
question_answer30) The moment of inertia of a uniform circular disc of radius R and mass M about an axis touching the disc at its diameter and normal to the disc is:
A)
\[M{{R}^{2}}\]
done
clear
B)
\[\frac{2}{5}M{{R}^{2}}\]
done
clear
C)
\[\frac{3}{2}M{{R}^{2}}\]
done
clear
D)
\[\frac{1}{2}M{{R}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer31) The momentum of a photon of energy 1 MeV in kg m/s, will be:
A)
\[0.33\times {{10}^{6}}\]
done
clear
B)
\[7\times {{10}^{-24}}\]
done
clear
C)
\[{{10}^{-22}}\]
done
clear
D)
\[5\times {{10}^{-22}}\]
done
clear
View Answer play_arrow
-
question_answer32) The radius of germanium (Ge) nuclide is measured to be twice the radius of \[_{4}^{9}Be\]. The number of nucleons in Ge are:
A)
73
done
clear
B)
74
done
clear
C)
75
done
clear
D)
72
done
clear
View Answer play_arrow
-
question_answer33) The molar specific heat at constant pressure of an ideal gas is (7/2)R. The ratio of specific heat at constant pressure to that at constant volume is:
A)
7/5
done
clear
B)
8/7
done
clear
C)
5/7
done
clear
D)
9/7
done
clear
View Answer play_arrow
-
question_answer34) The, earth is assumed to be a sphere of radius R, A platform is arranged at a height R from the surface of the earth. The escape velocity of a body from this platform is \[f{{v}_{e}}\], where \[{{v}_{e}}\] is its escape velocity from the surface of the earth. The value of f is:
A)
\[\sqrt{2}\]
done
clear
B)
\[\frac{1}{\sqrt{2}}\]
done
clear
C)
\[\frac{1}{3}\]
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
-
question_answer35) Two sound waves with wavelengths 5.0 m and 5.5 m respectively, each propagate in a gas with velocity 330 m/s. We expect the following number of beats per second:
A)
12
done
clear
B)
0
done
clear
C)
1
done
clear
D)
6
done
clear
View Answer play_arrow
-
question_answer36)
Power dissipated across the \[8\,\,\Omega \] resistor in the circuit shown here is 2 W. The power dissipated in watt units across the \[3\,\,\Omega \] resistor is: 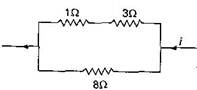
A)
2.0
done
clear
B)
1.0
done
clear
C)
0.5
done
clear
D)
3.0
done
clear
View Answer play_arrow
-
question_answer37) Kirchhoffs first and second laws for electrical circuits are consequences of:
A)
conservation of energy
done
clear
B)
conservation of electric charge and energy respectively
done
clear
C)
conservation of electric charge
done
clear
D)
conservation of energy and electric charge respectively
done
clear
View Answer play_arrow
-
question_answer38) A transverse wave propagating along x-axis is represented by: \[y\,(x,t)=8.0\,\sin \,\left( 0.5\,\pi x-4\pi t-\frac{\pi }{4} \right)\] where x is in metres and t is in seconds. The speed of the wave is:
A)
\[4\,\pi \,m/s\]
done
clear
B)
\[0.5\,\pi \,m/s\]
done
clear
C)
\[\frac{\pi }{4}\,m/s\]
done
clear
D)
8 m/s
done
clear
View Answer play_arrow
-
question_answer39) The time of reverberation of a room A is one second. What will be the time (in seconds) of reverberation of a room, having all the dimensions double of those of room A?
A)
2
done
clear
B)
4
done
clear
C)
\[\frac{1}{2}\]
done
clear
D)
1
done
clear
View Answer play_arrow
-
question_answer40) Which one of the following statements is true?
A)
Both light and sound waves in air are transverse
done
clear
B)
The sound waves in air are longitudinal while the light waves are transverse
done
clear
C)
Both light and sound waves in air are longitudinal
done
clear
D)
Both light and sound waves can travel in vacuum
done
clear
View Answer play_arrow
-
question_answer41) Above Curie temperature:
A)
a ferromagnetic substance becomes paramagnetic
done
clear
B)
a paramagnetic substance becomes diamagnetic
done
clear
C)
a diamagnetic substance becomes paramagnetic
done
clear
D)
a paramagnetic substance becomes ferromagnetic
done
clear
View Answer play_arrow
-
question_answer42) A convex lens and a concave lens, each having same focal length of 25 cm, are put in contact to form a combination of lenses. The powers diopters of the combination is:
A)
25
done
clear
B)
50
done
clear
C)
infinite
done
clear
D)
zero
done
clear
View Answer play_arrow
-
question_answer43) An electric dipole of moment \[\vec{p}\] is lying along a uniform electric field \[\vec{E}\]. The work done in rotating the dipole by \[{{90}^{\text{o}}}\] is.
A)
\[\sqrt{2}\,pE\]
done
clear
B)
\[\frac{pE}{2}\]
done
clear
C)
2pE
done
clear
D)
pE
done
clear
View Answer play_arrow
-
question_answer44) A parallel plate air capacitor is charged to a potential difference of V volts. After disconnecting the charging battery the distance between the plates of the capacitor is increased using an insulating handle. As a result the potential difference between the plates:
A)
decreases
done
clear
B)
does not change
done
clear
C)
becomes zero
done
clear
D)
increases
done
clear
View Answer play_arrow
-
question_answer45) A car runs at a constant speed on a circular tract of radius 100 m, taking 62.8 s for every circular lap. The average velocity and average speed for each circular lap respectively is:
A)
0, 0
done
clear
B)
0, 10 m/s
done
clear
C)
10 m/s, 10 m/s
done
clear
D)
10 m/s, 0
done
clear
View Answer play_arrow
-
question_answer46)
A square surface of side L m is in the plane of the paper. A uniform electric field \[\vec{E}\] (V/m), also in the plane of the paper, is limited only to the lower half of the square surface, (see figure). The electric flux in SI units associated with the surface is: 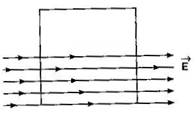
A)
\[E{{L}^{2}}/(2{{\varepsilon }_{0}})\]
done
clear
B)
\[E{{L}^{2}}/2\]
done
clear
C)
zero
done
clear
D)
\[E{{L}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer47) A tube of length L is filled completely with an incompressible liquid of mass M and closed at both the ends. The tube is then rotated in a horizontal plane about one of its ends with a uniform angular velocity \[\omega \]. The force exerted by the liquid at the other end is:
A)
\[\frac{ML{{\omega }^{2}}}{2}\]
done
clear
B)
\[\frac{M{{L}^{2}}\omega }{2}\]
done
clear
C)
\[ML{{\omega }^{2}}\]
done
clear
D)
\[\frac{M{{L}^{2}}{{\omega }^{2}}}{2}\]
done
clear
View Answer play_arrow
-
question_answer48)
A uniform rod of length \[l\] and mass m is free to rotate in a vertical plane about A. The rod initially in horizontal position is released. The initial angular acceleration of the rod is: (Moment of inertia of rod about A is \[\frac{m{{l}^{2}}}{3}\]) 
A)
\[\frac{3g}{2l}\]
done
clear
B)
\[\frac{2l}{3g}\]
done
clear
C)
\[\frac{3g}{2{{l}^{2}}}\]
done
clear
D)
\[mg\frac{l}{2}\]
done
clear
View Answer play_arrow
-
question_answer49) The vectors \[\vec{A}\] and \[\vec{B}\] are such that a: \[\left| \vec{A}+\vec{B} \right|=\left| \vec{A}-\vec{B} \right|\] The angle between the two vectors is:
A)
\[{{90}^{o}}\]
done
clear
B)
\[{{60}^{o}}\]
done
clear
C)
\[{{75}^{o}}\]
done
clear
D)
\[{{45}^{o}}\]
done
clear
View Answer play_arrow
-
question_answer50) Two bodies, A (of mass 1 kg) and B (of mass 3 kg) are dropped from heights of 16 m and 25 m, respectively. The ratio of the time taken by them to reach the ground is:
A)
5/4
done
clear
B)
12/5
done
clear
C)
5/12
done
clear
D)
4/5
done
clear
View Answer play_arrow
-
question_answer51) Identify the correct statement for change of Gibbs energy for a system \[(\Delta {{G}_{system}})\] at constant temperature and pressure:
A)
If \[\Delta {{G}_{system}}>0\], the process is spontaneous
done
clear
B)
If \[\Delta {{G}_{system}}=0\], the system has attained equilibrium
done
clear
C)
If \[\Delta {{G}_{system}}=0\], the system is still moving in a particular direction
done
clear
D)
If \[\Delta {{G}_{system}}<0\], the process is not spontaneous
done
clear
View Answer play_arrow
-
question_answer52) A solution containing 10 g per dm3 of urea (molecular mass \[=60\,\,g\,mo{{l}^{-1}}\]) is isotonic with a 5% solution of a non-volatile solute. The molecular mass of this non-volatile solute is:
A)
\[250\,\,g\,mo{{l}^{-1}}\]
done
clear
B)
\[300\,\,g\,mo{{l}^{-1}}\]
done
clear
C)
\[350\,\,g\,\,mo{{l}^{-1}}\]
done
clear
D)
\[200\,\,g\,\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer53) A plot of \[\log \,m/x\] versus \[\log \,\,p\] for the adsorption of a gas on a solid gives a straight line with slope equal to:
A)
- log k
done
clear
B)
n
done
clear
C)
\[\frac{1}{n}\]
done
clear
D)
log k
done
clear
View Answer play_arrow
-
question_answer54) Assume each reaction is carried out in an open container. For which reaction will \[\Delta H=\Delta E\]?
A)
\[{{H}_{2}}(g)\,+B{{r}_{2}}(g)\xrightarrow{\,}2HBr(g)\]
done
clear
B)
\[C(s)+2{{H}_{2}}O(g)\xrightarrow{\,}\,2{{H}_{2}}(g)\,+C{{O}_{2}}(g)\]
done
clear
C)
\[PC{{l}_{5}}(g)\,\xrightarrow{\,}\,PC{{l}_{3}}(g)\,+C{{l}_{2}}(g)\]
done
clear
D)
\[2CO(g)+{{O}_{2}}(g)\,\xrightarrow{\,}\,2C{{O}_{2}}(g)\]
done
clear
View Answer play_arrow
-
question_answer55) In a set of reactions propionic acid yielded a compound D. \[C{{H}_{3}}C{{H}_{2}}COO\xrightarrow[{}]{SOC{{l}_{2}}}B\xrightarrow[{}]{N{{H}_{3}}}C\xrightarrow[B{{r}_{2}}]{KOH}D\] The structure of D would be:
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}CON{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}NHC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer56) During the process of digestion, the proteins present in food materials are hydrolysed to amino acids. The two enzymes involved in the process Proteins \[\xrightarrow{Enzyme\,\,(A)}\]Polypeptides \[\xrightarrow{Enzyme\,(B)}\,\text{Amino}\,\text{acids},\] are respectively:
A)
amylase and maltase
done
clear
B)
diastase and lipase
done
clear
C)
pepsin and trypsin
done
clear
D)
invertase and zymase
done
clear
View Answer play_arrow
-
question_answer57) The human body does not produce:
A)
DNA
done
clear
B)
vitamins
done
clear
C)
hormones
done
clear
D)
enzymes
done
clear
View Answer play_arrow
-
question_answer58) CsBr crystallises in a body centred cubic lattice. The unit cell length is 436.6 pm. Given that the atomic mass of Cs = 133 and that of Br = 80 amu and Avogadro number being \[6.02\times {{10}^{23}}\,mo{{l}^{-1}},\] the density of \[CsBr\] is:
A)
\[42.5\,g/c{{m}^{3}}\]
done
clear
B)
\[0.425\,g/\,c{{m}^{3}}\]
done
clear
C)
\[8.25\,g/\,c{{m}^{3}}\]
done
clear
D)
\[4.25\,g/c{{m}^{3}}\]
done
clear
View Answer play_arrow
-
question_answer59) More number of oxidation states are exhibited by the actinoids than by the lanthanoids. The main reason for this is:
A)
more energy difference between 5f and 6d orbitals than that between 4f and 5d orbitals
done
clear
B)
lesser energy difference between 5f and 6d orbitals than that between 4f and 5d orbitals
done
clear
C)
greater metallic character of the lanthanoids than that of the corresponding actinoids
done
clear
D)
more active nature of the actinoids
done
clear
View Answer play_arrow
-
question_answer60) Given: The mass of electron is \[9.11\times {{10}^{-31}}kg\] Planck constant is \[6.626\,\times {{10}^{-34}}Js,\] the uncertainty involved in the measurement of velocity within a distance of 0.1 \[\overset{\text{o}}{\mathop{\text{A}}}\,\] is :
A)
\[5.79\times {{10}^{6}}m{{s}^{-1}}\]
done
clear
B)
\[5.79\times 10\,m{{s}^{-1}}\]
done
clear
C)
\[5.79\times {{10}^{8}}\,m{{s}^{-1}}\]
done
clear
D)
\[579\,\times {{10}^{5}}\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer61) Copper sulphate dissolves in excess of KCN to give:
A)
CuCN
done
clear
B)
\[{{[Cu{{(CN)}_{4}}]}^{3-}}\]
done
clear
C)
\[{{[Cu{{(CN)}_{4}}]}^{2-}}\]
done
clear
D)
\[Cu\,{{(CN)}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer62) In which of the following pairs are both the ions coloured in aqueous solution?
A)
\[N{{i}^{2+}},\,T{{i}^{3+}}\] \[_{28}Ni=1{{s}^{2}},2{{s}^{2}}\,2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{8}},4{{s}^{2}}\]
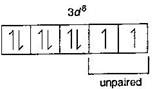
\[N{{i}^{2+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{{}}}\] \[_{22}Ti=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{2}},4{{s}^{2}}\]
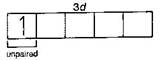
\[T{{i}^{3+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{1}}\] \[_{21}Sc=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{1}},4{{s}^{2}}\] \[S{{c}^{3+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}\] (unpaired electron in d-orbital is not possible) \[_{29}Cu=1{{s}^{2}},2{{s}^{2}}\,2{{p}^{6}},3{{s}^{2}}\,3{{p}^{6}}\,3{{d}^{10}},4{{s}^{1}}\] \[C{{u}^{+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}\] (complete d-orbital) Hence, in above ions, \[N{{i}^{2+}}\] and \[T{{i}^{3+}}\] ions are coloured ions in aqueous solution due to presence of unpaired electrons in d-sub-shell.
B)
\[S{{c}^{3+}},\,T{{i}^{3+}}\]
done
clear
C)
\[S{{c}^{3}},\,C{{o}^{2+}}\]
done
clear
D)
\[N{{i}^{2+}},\,C{{u}^{+}}\] (At. no.: Sc = 21, Ti = 22, Ni = 28, Cu = 29, Co = 27)
done
clear
View Answer play_arrow
-
question_answer63) \[A{{l}_{2}}{{O}_{3}}\] can be converted to anhydrous \[AlC{{l}_{3}}\] by heating:
A)
\[A{{l}_{2}}{{O}_{3}}\] with HCl gas
done
clear
B)
\[A{{l}_{2}}{{O}_{3}}\] with NaCl in solid state
done
clear
C)
a mixture of \[A{{l}_{2}}{{O}_{3}}\] and carbon in dry \[C{{l}_{2}}\] gas
done
clear
D)
\[A{{l}_{2}}{{O}_{3}}\] with \[C{{l}_{2}}\] gas
done
clear
View Answer play_arrow
-
question_answer64) The enthalpy and entropy change for the reaction: \[B{{r}_{2}}(l)+C{{l}_{2}}(g)\to 2BrCl(g)\] are \[30\,kJ\,mo{{l}^{-1}}\] and \[105\,\,J{{K}^{-1}}\,mo{{l}^{-1}}\] respectively. The temperature at which the reaction will be in equilibrium is:
A)
285.7 K
done
clear
B)
273 K
done
clear
C)
450 K
done
clear
D)
300 K
done
clear
View Answer play_arrow
-
question_answer65) The appearance of colour in solid alkali metal halides is generally due to:
A)
F-centres
done
clear
B)
Schottky defect
done
clear
C)
Frenkel defect
done
clear
D)
Interstitial positions
done
clear
View Answer play_arrow
-
question_answer66) The general molecular formula, which represents the homologous series of alkanols is:
A)
\[{{C}_{n}}{{H}_{2n}}{{O}_{2}}\]
done
clear
B)
\[{{C}_{n}}{{H}_{2n}}O\]
done
clear
C)
\[{{C}_{n}}{{H}_{2n+1}}O\]
done
clear
D)
\[{{C}_{n}}{{H}_{2n+2}}O\]
done
clear
View Answer play_arrow
-
question_answer67) If \[E_{F{{e}^{2+}}/Fe}^{o}=-0.441\,V\,and\] \[E_{F{{e}^{3+}}/F{{e}^{2+}}}^{o}=0.771\,V\], the standard emf of the reaction: \[Fe+2F{{e}^{3+}}\to 3F{{e}^{2+}}\] will be:
A)
0.330 V
done
clear
B)
1.653 V
done
clear
C)
1.212 V
done
clear
D)
0.111 V
done
clear
View Answer play_arrow
-
question_answer68) For the reaction \[2A+B\to 3C+D\] which of the following does not express the reaction rate?
A)
\[-\frac{d\,[C]}{3\,dt}\]
done
clear
B)
\[-\frac{d\,[B]}{dt}\]
done
clear
C)
\[\frac{d\,[D]}{dt}\]
done
clear
D)
\[-\frac{d\,[A]}{2\,dt}\]
done
clear
View Answer play_arrow
-
question_answer69) For the reaction, \[C{{H}_{4}}(g)+2{{O}_{2}}(g)\rightleftharpoons C{{O}_{2}}(g)+2{{H}_{2}}O(\ell )\,,\] \[{{\Delta }_{r}}H=-170.8\,kJ\,mo{{l}^{-1}}\] Which of the following statements is not true?
A)
At equilibrium, the concentrations of \[C{{O}_{2}}(g)\] and \[{{H}_{2}}O(l)\] are not equal
done
clear
B)
The equilibrium constant for the reaction is given by \[{{K}_{p}}=\frac{[C{{O}_{2}}]}{[C{{H}_{4}}][{{O}_{2}}]}\]
done
clear
C)
Addition of \[C{{H}_{4}}(g)\] or \[{{O}_{2}}(g)\] at equilibrium will cause a shift to the right
done
clear
D)
The reaction is exothermic
done
clear
View Answer play_arrow
-
question_answer70) \[{{[NH(C{{H}_{2}})NHCO{{(C{{H}_{2}})}_{4}}CO]}_{n}}\] is a:
A)
co-polymer
done
clear
B)
addition polymer
done
clear
C)
thermo-setting polymer
done
clear
D)
homopolymer
done
clear
View Answer play_arrow
-
question_answer71) A carbonyl compound reacts with hydrogen cyanide to form cyanohydrin which on hydrolysis forms a racemic mixture of \[\alpha \]-hydroxy acid. The carbonyl compound is:
A)
acetaldehyde
done
clear
B)
acetone
done
clear
C)
diethyl ketone
done
clear
D)
formaldehyde
done
clear
View Answer play_arrow
-
question_answer72) Which one of the following is a peptide hormone?
A)
Glucagon
done
clear
B)
Testosterone
done
clear
C)
Thyroxin
done
clear
D)
Adrenaline
done
clear
View Answer play_arrow
-
question_answer73) The major organic product in the reaction, \[C{{H}_{3}}-O-CH{{(C{{H}_{3}})}_{2}}+HI\to \] Product is:
A)
\[C{{H}_{3}}OH+{{(C{{H}_{3}})}_{2}}CHI\]
done
clear
B)
\[IC{{H}_{2}}OCH{{(C{{H}_{3}})}_{2}}\]
done
clear
C)
\[C{{H}_{3}}O\,\,\underset{\begin{smallmatrix} | \\ I \end{smallmatrix}}{\mathop{C}}\,{{(C{{H}_{3}})}_{2}}\]
done
clear
D)
\[C{{H}_{3}}I+{{(C{{H}_{3}})}_{2}}CHOH\]
done
clear
View Answer play_arrow
-
question_answer74) Nucleophilic addition reaction will be most favoured in:
A)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}C=O\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
D)
\[C{{H}_{3}}CHO\]
done
clear
View Answer play_arrow
-
question_answer75) The enthalpy of combustion of \[{{H}_{2}}\], cyclohexene \[({{C}_{6}}{{H}_{10}})\] and cyclohexene \[({{C}_{6}}{{H}_{12}})\] are -241, -3800 and -3920 kJ per mol respectively. Heat of hydrogenation of cyclohexene is:
A)
-121 kJ per mol
done
clear
B)
+121 kJ per mol
done
clear
C)
+242 kJ per mol
done
clear
D)
-242 kJ per mol
done
clear
View Answer play_arrow
-
question_answer76) Self condensation of two moles of ethyl acetate in presence of sodium ethoxide yields:
A)
ethyl butyrate
done
clear
B)
acetoacetic ester
done
clear
C)
methyl acetoacetate
done
clear
D)
ethyl propionate
done
clear
View Answer play_arrow
-
question_answer77) Consider the reaction \[{{N}_{2}}(g)+3{{H}_{2}}(g)\xrightarrow{{}}2N{{H}_{3}}(g)\] The equality relationship between \[\frac{d[N{{H}_{3}}]}{dt}\] and \[-\frac{d[{{H}_{2}}]}{dt}\] is:
A)
\[\frac{d\,[N{{H}_{3}}]}{dt}=-\frac{1}{3}\,\frac{d\,[{{H}_{2}}]}{dt}\]
done
clear
B)
\[+\frac{d\,[N{{H}_{3}}]}{dt}=-\frac{2}{3}\,\frac{d\,[{{H}_{2}}]}{dt}\]
done
clear
C)
\[+\frac{d\,[N{{H}_{3}}]}{dt}=-\frac{3}{2}\,\frac{d\,[{{H}_{2}}]}{dt}\]
done
clear
D)
\[\frac{d\,[N{{H}_{3}}]}{dt}=-\,\frac{d\,[{{H}_{2}}]}{dt}\]
done
clear
View Answer play_arrow
-
question_answer78) Which of the following is not chiral?
A)
2-butanol
done
clear
B)
2, 3-dibromopentane
done
clear
C)
3-bromopentane
done
clear
D)
2-hydroxypropanoic acid
done
clear
View Answer play_arrow
-
question_answer79) \[[Co{{(N{{H}_{3}})}_{4}}{{(N{{O}_{2}})}_{2}}\,]Cl\] exhibit its:
A)
linkage isomerism, ionization isomerism and optical isomerism
done
clear
B)
linkage isomerism, ionization isomerism and geometrical isomerism
done
clear
C)
ionization isomerism, geometrical isomerism and optical isomerism
done
clear
D)
linkage isomerism, geometrical isomerism and optical isomerism
done
clear
View Answer play_arrow
-
question_answer80) \[[Cr{{({{H}_{2}}O)}_{6}}]C{{l}_{3}}\] (at. no. of Cr = 24) has a magnetic moment of 3.83 BM, the correct distribution of 3d electrons in die chromium of the complex is:
A)
\[3d_{{{x}^{2}}-{{y}^{2}}}^{1},\,3d_{{{z}^{2}}}^{1},\,3d_{xz}^{1}\]
done
clear
B)
\[3d_{xy}^{1},\,3d_{{{x}^{2}}-{{y}^{2}}}^{1},\,3d_{yz}^{1}\]
done
clear
C)
\[3d_{xy}^{1},\,3d_{zy}^{1},\,3d_{xz}^{1}\]
done
clear
D)
\[3d_{xy}^{1},\,3d_{yz}^{1},\,3d_{{{z}^{2}}}^{1}\]
done
clear
View Answer play_arrow
-
question_answer81) 1.00 g of a non-electrolyte solute (molar mass \[250g\,mo{{l}^{-1}}\]) was dissolved in 51.2 g of benzene. If the freezing point depression constant, \[{{K}_{f}}\] of benzene is \[5.12\,K\,kg\,mo{{l}^{-1}},\] the freezing point of benzene will be lowered by:
A)
0.4 K
done
clear
B)
0.3 K
done
clear
C)
0.5 K
done
clear
D)
0.2 K
done
clear
View Answer play_arrow
-
question_answer82) Which of the following pairs constitutes a buffer?
A)
\[HN{{O}_{2}}\] and \[NaN{{O}_{2}}\]
done
clear
B)
NaOH and NaCl
done
clear
C)
\[HN{{O}_{3}}\] and \[N{{H}_{4}}N{{O}_{3}}\]
done
clear
D)
HCl and KCl
done
clear
View Answer play_arrow
-
question_answer83) The hydrogen ion concentration of a \[{{10}^{-8}}M\,\,HCl\] aqueous solution at \[298\,\,K({{K}_{w}}={{10}^{-14}})\] is:
A)
\[1.0\times {{10}^{-6}}M\]
done
clear
B)
\[1.0525\,\times {{10}^{-7}}\,M\]
done
clear
C)
\[9.525\,\times {{10}^{-8}}\,M\]
done
clear
D)
\[1.0\times {{10}^{-8}}M\]
done
clear
View Answer play_arrow
-
question_answer84) A solution of acetone in ethanol:
A)
shows a negative deviation from Raoult's law
done
clear
B)
shows a positive deviation from Raoult's law
done
clear
C)
behaves like a near ideal solution
done
clear
D)
obeys Raoult?s law
done
clear
View Answer play_arrow
-
question_answer85) A hypothetical electrochemical cell is shown below \[A|{{A}^{+}}(xM)||{{B}^{+}}(yM)|B\] The emf measured is + 0.20 V. The cell reaction is:
A)
\[{{A}^{+}}+B\xrightarrow{{}}A+{{B}^{+}}\]
done
clear
B)
\[{{A}^{+}}+{{e}^{-}}\xrightarrow{{}}A;\,{{B}^{+}}+{{e}^{-}}\to B\]
done
clear
C)
the cell reaction cannot be predicted
done
clear
D)
\[A+{{B}^{+}}\xrightarrow{{}}{{A}^{+}}+B\]
done
clear
View Answer play_arrow
-
question_answer86) Ethylene oxide when treated with Grignard reagent yields:
A)
secondary alcohol
done
clear
B)
tertiary alcohol
done
clear
C)
cyclopropyl alcohol
done
clear
D)
primary alcohol
done
clear
View Answer play_arrow
-
question_answer87) During osmosis, flow of water through a semi-permeable membrane is:
A)
from solution having higher concentration only
done
clear
B)
from both sides of semi-permeable membrane with equal flow rates
done
clear
C)
from both sides of semi-permeable membrane with unequal flow rates
done
clear
D)
from solution having lower concentration only
done
clear
View Answer play_arrow
-
question_answer88) Which of the following is more basic than aniline?
A)
Diphenylamine
done
clear
B)
Triphenylamine
done
clear
C)
p-nitroaniline
done
clear
D)
Benzyiamine
done
clear
View Answer play_arrow
-
question_answer89) In which of the following molecules are all the bonds not equal?
A)
\[Cl{{F}_{3}}\]
done
clear
B)
\[B{{F}_{3}}\]
done
clear
C)
\[Al{{F}_{3}}\]
done
clear
D)
\[N{{F}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer90) The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of \[N{{H}_{3}}\] (1.5 D) is larger than that of \[N{{F}_{3}}\] (0.2 D). This is because:
A)
in \[N{{H}_{3}}\] as well as in \[N{{F}_{3}}\] the atomic dipole and bond dipole are in the same direction
done
clear
B)
in \[N{{H}_{3}}\] the atomic dipole and bond dipole are in the same direction whereas in \[N{{F}_{3}}\] these are in opposite directions
done
clear
C)
in \[N{{H}_{3}}\] as well as \[N{{F}_{3}}\] the atomic dipole and bond dipole are in opposite directions
done
clear
D)
in \[N{{H}_{3}}\] the atomic dipole and bond dipole are in the opposite directions whereas in \[N{{F}_{3}}\] these are in the same directions
done
clear
View Answer play_arrow
-
question_answer91) The correct order of the mobility of the alkali metal ions in aqueous solution is:
A)
\[L{{i}^{+}}>N{{a}^{+}}>{{K}^{+}}>R{{b}^{+}}\]
done
clear
B)
\[N{{a}^{+}}>{{K}^{+}}>R{{b}^{+}}>L{{i}^{+}}\]
done
clear
C)
\[{{K}^{+}}>R{{b}^{+}}>N{{a}^{+}}>L{{i}^{+}}\]
done
clear
D)
\[R{{b}^{+}}>{{K}^{+}}>N{{a}^{+}}>L{{i}^{+}}\]
done
clear
View Answer play_arrow
-
question_answer92) The correct order regarding the electronegativity of hybrid orbitals of carbon is:
A)
\[sp>s{{p}^{2}}<s{{p}^{3}}\]
done
clear
B)
\[sp>s{{p}^{2}}>s{{p}^{3}}\]
done
clear
C)
\[sp<s{{p}^{2}}>s{{p}^{3}}\]
done
clear
D)
\[sp<s{{p}^{2}}<s{{p}^{3}}\]
done
clear
View Answer play_arrow
-
question_answer93) Which of the following species has a linear shape?
A)
\[NO_{2}^{-}\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
\[NO_{2}^{+}\]
done
clear
D)
\[{{O}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer94) Which of the following is the most basic oxide?
A)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
B)
\[S{{b}_{2}}{{O}_{3}}\]
done
clear
C)
\[B{{i}_{2}}{{O}_{3}}\]
done
clear
D)
\[Se{{O}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer95) The orientation of an atomic orbital is governed by:
A)
azimuthal quantum number
done
clear
B)
spin quantum number
done
clear
C)
magnetic quantum number
done
clear
D)
principal quantum number
done
clear
View Answer play_arrow
-
question_answer96) Which of the following is not a correct statement?
A)
The electron-deficient molecules can act as Lewis acids
done
clear
B)
The canonical structures have no real existence
done
clear
C)
Every \[A{{B}_{5}}\] molecule does in fact have square pyramid structure
done
clear
D)
Multiple bonds are always shorter than corresponding single bonds
done
clear
View Answer play_arrow
-
question_answer97) The number of unpaired electrons in a paramagnetic diatomic molecule of an element with atomic number 16 is:
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
1
done
clear
View Answer play_arrow
-
question_answer98) Which one of the following orders is not in accordance with the property stated against it?
A)
\[{{F}_{2}}>C{{l}_{2}}>B{{r}_{2}}>{{l}_{2}}\]: Oxidising power
done
clear
B)
HI > HBr > HCI > HF: Acidic property in water
done
clear
C)
\[{{F}_{2}}>C{{l}_{2}}>B{{r}_{2}}>{{l}_{2}}\]: Electronegativity
done
clear
D)
\[{{F}_{2}}>C{{l}_{2}}>B{{r}_{2}}>{{l}_{2}}\]: Bond dissociation energy
done
clear
View Answer play_arrow
-
question_answer99) Which of the following is not isostructural with \[SiC{{l}_{4}}\]?
A)
\[SC{{l}_{4}}\]
done
clear
B)
\[SO_{4}^{2-}\]
done
clear
C)
\[PO_{4}^{3-}\]
done
clear
D)
\[NH_{4}^{+}\]
done
clear
View Answer play_arrow
-
question_answer100)
The IUPAC name of 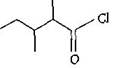 is:
is:
A)
3, 4-dimethylpentanoyl chloride
done
clear
B)
1-chloro-1-oxo-2, 3-dimethylpentane
done
clear
C)
2-ethyl-3-methyIbutanoyi chloride
done
clear
D)
2, 3-dimethylpentanoyl chloride
done
clear
View Answer play_arrow
-
question_answer101) What would be the number of chromosomes in the cells of the aleurone layer in a plant species with 8 chromosomes in its synergids?
A)
16
done
clear
B)
24
done
clear
C)
32
done
clear
D)
8
done
clear
View Answer play_arrow
-
question_answer102) Pineapple (annanas) fruit develops from:
A)
a unilocular polycarpillary flower
done
clear
B)
a multipistillate syncarpous flower
done
clear
C)
a cluster of compactly borne flowers on a common axis
done
clear
D)
a multilocular monocarpillary flower
done
clear
View Answer play_arrow
-
question_answer103) Golden rice is a promising transgenic crop. When released for cultivation, it will help in:
A)
alleviation of vitamin- A deficiency
done
clear
B)
pest resistance
done
clear
C)
herbicide tolerance
done
clear
D)
producing a petrol-like fuel from rice
done
clear
View Answer play_arrow
-
question_answer104) Parthenocarpic tomato fruits can be produced by:
A)
removing androecium of flowers before pollen grains are released
done
clear
B)
treating the plants with low concentrations of gibberellic acid and auxins
done
clear
C)
raising the plants from vernalized seeds
done
clear
D)
treating the plants with phenylmercuric acetate
done
clear
View Answer play_arrow
-
question_answer105) How does pruning help in making the hedge dense?
A)
It induces the differentiation of new shoots from the rootstock
done
clear
B)
It frees axillary buds from apical dominance
done
clear
C)
The apical shoot grows faster after pruning
done
clear
D)
It releases wound hormones
done
clear
View Answer play_arrow
-
question_answer106) The ?blue baby? syndrome results from:
A)
excess of chloride
done
clear
B)
methaemoglobin
done
clear
C)
excess of dissolved oxygen
done
clear
D)
excess of TDS (Total Dissolved Solids)
done
clear
View Answer play_arrow
-
question_answer107) Praying mentis is a good example of:
A)
mullerian mimicry
done
clear
B)
warning colouration
done
clear
C)
social insects
done
clear
D)
camouflage
done
clear
View Answer play_arrow
-
question_answer108) Which one of the following statements is correct?
A)
Neurons regulate endocrine activity, but not vice versa
done
clear
B)
Endocrine glands regulate neural activity and nervous system regulates endocrine glands
done
clear
C)
Neither hormones control neural activity nor the neurons control endocrine activity
done
clear
D)
Endocrine glands regulate neural activity, but not vice versa
done
clear
View Answer play_arrow
-
question_answer109) Examination of blood of a person suspected of having anaemia, shows large, immature, nucleated erythrocytes without haemoglobin. Supplementing his diet with which of the following, is likely to alleviate his symptoms?
A)
Thiamine
done
clear
B)
Folic acid and cobalamine
done
clear
C)
Riboflavin
done
clear
D)
Iron compounds
done
clear
View Answer play_arrow
-
question_answer110) Farmers in a particular region were concerned that pre-mature yellowing of leaves of a pulse crop might cause decrease in the yield. Which treatment could be most beneficial to obtain maximum seed yield?
A)
Frequent irrigation of the crop
done
clear
B)
Treatment of the paints with cytokinins along with a small dose of nitrogenous fertilizer
done
clear
C)
Removal of all yellow leaves and spraying the remaining green leaves with 2, 4, 5-trichlorophenoxy acetic acid
done
clear
D)
Application of iron and magnesium to promote synthesis of chlorophyll
done
clear
View Answer play_arrow
-
question_answer111) In which of the following fruits is the edible part the aril?
A)
Custard apple
done
clear
B)
Pomegranate
done
clear
C)
Orange
done
clear
D)
Litchi
done
clear
View Answer play_arrow
-
question_answer112) Which one of the following aminoacids was not found to be synthesized in Miller's experiment?
A)
Glycine
done
clear
B)
Asparticacid
done
clear
C)
Glutamic acid
done
clear
D)
Alanine
done
clear
View Answer play_arrow
-
question_answer113) Crop plants grown in monoculture are:
A)
low in yield
done
clear
B)
free from intraspecific competition
done
clear
C)
characterised by poor root system
done
clear
D)
highly prone to pests
done
clear
View Answer play_arrow
-
question_answer114) Montreal protocol which calls for appropriate action to protect the ozone layer from human activities was passed in the year:
A)
1986
done
clear
B)
1987
done
clear
C)
1988
done
clear
D)
1985
done
clear
View Answer play_arrow
-
question_answer115) The formula for exponential population growth is:
A)
dt/dN = rN
done
clear
B)
dN/rN = dt
done
clear
C)
rN/dN = dt
done
clear
D)
dN/dt = Rn
done
clear
View Answer play_arrow
-
question_answer116) Which one of the following is not used for construction of ecological pyramids?
A)
Dry weight
done
clear
B)
Number of individuals
done
clear
C)
Rate of energy flow
done
clear
D)
Fresh weight
done
clear
View Answer play_arrow
-
question_answer117) Niche overlap indicates:
A)
active co-operation between two species
done
clear
B)
two different parasites on the same host
done
clear
C)
sharing of one or more resources between the two species
done
clear
D)
mutualism between two species
done
clear
View Answer play_arrow
-
question_answer118) In photosystem-I, the first electron acceptor is:
A)
ferredoxin
done
clear
B)
cytochrome
done
clear
C)
plastocyanin
done
clear
D)
an iron-sulphur protein
done
clear
View Answer play_arrow
-
question_answer119) Treatment of seed at low temperature under moist conditions to break its dormancy is called:
A)
scarification
done
clear
B)
vernalization
done
clear
C)
chelation
done
clear
D)
stratification
done
clear
View Answer play_arrow
-
question_answer120) Which one of the following is the most suitable, medium for culture of DrosopMLa melanogaster?
A)
Moist bread
done
clear
B)
Agar agar
done
clear
C)
Ripe banana
done
clear
D)
Cow dung
done
clear
View Answer play_arrow
-
question_answer121) Which one of the following is not included under in situ conservation?
A)
Sanctuary
done
clear
B)
Botanical garden
done
clear
C)
Biosphere reserve
done
clear
D)
National park
done
clear
View Answer play_arrow
-
question_answer122) Which antibiotic inhibits interaction between t-RNA and m-RNA during bacterial protein synthesis?
A)
Erythromycin
done
clear
B)
Neomycin
done
clear
C)
Streptomycin
done
clear
D)
Tetracycline
done
clear
View Answer play_arrow
-
question_answer123) Phenotype of an organism is the result of:
A)
mutations and linkages
done
clear
B)
cytoplasmic effects and nutrition
done
clear
C)
environmental changes and sexual dimorphism
done
clear
D)
genotype and environment interactions
done
clear
View Answer play_arrow
-
question_answer124) Photochemical smog pollution does not contain:
A)
ozone
done
clear
B)
nitrogen dioxide
done
clear
C)
carbon dioxide
done
clear
D)
PAN (Peroxy Acyl Nitrate)
done
clear
View Answer play_arrow
-
question_answer125) Moss peat is used as a packing material for sending flowers and live plants to distant places because:
A)
it is easily available
done
clear
B)
it is hygroscopic
done
clear
C)
it reduces transpiration
done
clear
D)
it serves as a disinfectant
done
clear
View Answer play_arrow
-
question_answer126) A common structural feature of vessel elements and sieve tube elements is:
A)
thick secondary walls
done
clear
B)
pores on lateral walls
done
clear
C)
presence of P-protein
done
clear
D)
enucleate condition
done
clear
View Answer play_arrow
-
question_answer127) The thalloid body of a slime mould (Myxomycetes) is known as:
A)
protonema
done
clear
B)
Plasmodium
done
clear
C)
fruiting body
done
clear
D)
mycelium
done
clear
View Answer play_arrow
-
question_answer128) In which mode of inheritance do you expect more maternal influence among the offspring?
A)
Autosomal
done
clear
B)
Cytoplasmic
done
clear
C)
Y-linked
done
clear
D)
X-linked
done
clear
View Answer play_arrow
-
question_answer129) What type of placentation is seen in sweet pea?
A)
Basal
done
clear
B)
Axile
done
clear
C)
Free central
done
clear
D)
Marginal
done
clear
View Answer play_arrow
-
question_answer130) Long filamentous threads protruding at die end of a young cob of maize are:
A)
anthers
done
clear
B)
styles
done
clear
C)
ovaries
done
clear
D)
hairs
done
clear
View Answer play_arrow
-
question_answer131) Conifers differ from grasses in the:
A)
production of seeds from ovules
done
clear
B)
lack of xylem tracheids
done
clear
C)
absence of pollen tubes
done
clear
D)
formation of endosperm before fertilization
done
clear
View Answer play_arrow
-
question_answer132) How many different kinds of gametes will be produced by a plant having the genotype AABbCC?
A)
Three
done
clear
B)
Four
done
clear
C)
Nine
done
clear
D)
Two
done
clear
View Answer play_arrow
-
question_answer133) In maize, hybrid vigour is exploited by:
A)
bombarding the protoplast with ONA
done
clear
B)
crossing of two inbreed parental lines
done
clear
C)
harvesting seeds from the most productive plants
done
clear
D)
inducing mutations
done
clear
View Answer play_arrow
-
question_answer134) Which of the following statements regarding mitochondrial membrane is not correct?
A)
The outer membrane is permeable to all kinds of molecules
done
clear
B)
The enzymes of the electron transfer chain are embedded in the outer membrane
done
clear
C)
The inner membrane is highly convoluted forming a series of infoldings
done
clear
D)
The outer membrane resembles a sieve
done
clear
View Answer play_arrow
-
question_answer135) Amino acid sequence, in protein synthesis is decided by the sequence of:
A)
t-RNA
done
clear
B)
m-RNA
done
clear
C)
c-DNA
done
clear
D)
r-RNA
done
clear
View Answer play_arrow
-
question_answer136) How many ATP molecules could maximally be generated from one molecule of glucose, if the complete oxidation of one mole of glucose to \[C{{O}_{2}}\]and \[{{H}_{2}}O\] yields 686 kcal and the useful chemical energy available in the high energy phosphate bond of one mole of ATP is 12 kcal?
A)
Two
done
clear
B)
Thirty
done
clear
C)
Fifty seven
done
clear
D)
One
done
clear
View Answer play_arrow
-
question_answer137) An organic substance bound to an enzyme and essential for its acvity is called:
A)
coenzyme
done
clear
B)
holoenzyme
done
clear
C)
apoenzyme
done
clear
D)
isoenzyme
done
clear
View Answer play_arrow
-
question_answer138) Bowman's glands are found in:
A)
olfactory epithelium
done
clear
B)
external auditory canal
done
clear
C)
cortical nephrons only
done
clear
D)
juxtamedullary nephrons
done
clear
View Answer play_arrow
-
question_answer139) The bacterium (Clostridium botulinum) that causes botulism is:
A)
a facultative anaerobe
done
clear
B)
an obligate anaerobe
done
clear
C)
a facultative aerobe
done
clear
D)
an obligate aerobe
done
clear
View Answer play_arrow
-
question_answer140) Which one of the following is the correctly matched pair of an endangered animal and a National Park?
A)
lion - Corbett National Park
done
clear
B)
Rhinoceros - Kaziranga National Park
done
clear
C)
Wild ass - Dudhwa National Park
done
clear
D)
Great Indian - Keoladeo National Park bustard
done
clear
View Answer play_arrow
-
question_answer141) A person showing upredictable moods, outbursts of emotion, quarrelsome behaviour and conflicts with others is suffering from:
A)
schizophrenia
done
clear
B)
borderline personality disorder (BPD)
done
clear
C)
mood disorders
done
clear
D)
addictive disorders
done
clear
View Answer play_arrow
-
question_answer142) Sulphur is an important nutrient for optimum growth and productivity in:
A)
pulse crops
done
clear
B)
cereals
done
clear
C)
fibre crops
done
clear
D)
oilseed crops
done
clear
View Answer play_arrow
-
question_answer143) Pentamerous, actinomorphic flowers, bicarpillary ovary with oblique septa, and fruit a capsule or berry, are characteristic features of:
A)
Asteraceae
done
clear
B)
Brassicaceae
done
clear
C)
Solanaceae
done
clear
D)
Liliaceae
done
clear
View Answer play_arrow
-
question_answer144) In a moss the sporophyte:
A)
is partially parasitic on the gametophyte
done
clear
B)
produces gametes that give rise to the gametophyte
done
clear
C)
arises from a spore produced from the gametophyte
done
clear
D)
manufactures food for itself, as well as for the gametophyte
done
clear
View Answer play_arrow
-
question_answer145) Curing of tea leaves is brought about by the activity of:
A)
bacteria
done
clear
B)
mycorrhiza
done
clear
C)
viruses
done
clear
D)
fungi
done
clear
View Answer play_arrow
-
question_answer146) People living at sea level have around 5 million RBC per cubic millimeter of their blood whereas those living at an altitude of 5400 metres have around 8 million. This is because at high altitude:
A)
people get pollution-free air to breathe and more oxygen is available
done
clear
B)
atmospheric \[{{O}_{2}}\] level is less and hence more RBCs are needed to absorb the required amount of \[{{O}_{2}}\] to survive
done
clear
C)
there is more UV radiation which enhances RBC production
done
clear
D)
people eat more nutritive food, therefore more RBCs are formed
done
clear
View Answer play_arrow
-
question_answer147) An important evidence in favour of organic evolution is the occurrence of:
A)
homologous and vestigial organs
done
clear
B)
analogous and vestigial organs
done
clear
C)
homologous organs only
done
clear
D)
homologous and analogous organs
done
clear
View Answer play_arrow
-
question_answer148) Which one of the following is not a living fossil?
A)
King crab
done
clear
B)
Sphenodon
done
clear
C)
Archaeopteryx
done
clear
D)
Peripatus
done
clear
View Answer play_arrow
-
question_answer149) Annual migration does not occur in the case of:
A)
salmon
done
clear
B)
Siberian crane
done
clear
C)
salamander
done
clear
D)
arctic tem
done
clear
View Answer play_arrow
-
question_answer150) A major breakthrough in the studies of cells came with the development of electron microscope. This is because:
A)
the resolution power of the electron microscope is much higher than that of the light microscope
done
clear
B)
the resolving power of the electron microscope is 200 - 350 nm as compared to 0.1 - 0.2 nm for the light microscope
done
clear
C)
electron beam can pass through thick materials, whereas light microscopy requires thin sections
done
clear
D)
the electron microscope is more powerful than the light microscope as it uses a beam of electrons which has wavelength much longer than that of photons
done
clear
View Answer play_arrow
-
question_answer151) Which one of the following is a matching set of a phylum and its three examples?
A)
Cnidaria?Bonellia, Physalia, Aurelia
done
clear
B)
Platyhelminthes ? Planaria, Schistosoma, Enterobius
done
clear
C)
Mollusca ? Loligo, Teredo, Octopus
done
clear
D)
Porifera ? Spongilla, Euplectella, pennatula
done
clear
View Answer play_arrow
-
question_answer152) Metameric segmentation is the characteristic of:
A)
Platyhelminthes and Arthropoda
done
clear
B)
Echinodermata and Annelida
done
clear
C)
Annelida and Arthropoda
done
clear
D)
Mollusca and Chordata
done
clear
View Answer play_arrow
-
question_answer153) Which of the following pairs of an animal and a plant represents endangered organisms in India?
A)
Bentinckia nicobarica and red panda
done
clear
B)
Tamarind and rhesus monkey
done
clear
C)
Cinchona and leopard
done
clear
D)
Banyan and black buck
done
clear
View Answer play_arrow
-
question_answer154) Jurassic period of the Mesozoic era is characterised by:
A)
gymnosperms are dominant plants and first birds appear
done
clear
B)
radiation of reptiles and origin of mammal like reptiles
done
clear
C)
dinosaurs become extinct and angiosperms appear
done
clear
D)
flowering plants and first dinosaurs appear
done
clear
View Answer play_arrow
-
question_answer155) What is common about Trypanosoma, Noctiluca, Monocystis and Giardia?
A)
These are all unicellular protists
done
clear
B)
They have flagella
done
clear
C)
They produce spores
done
clear
D)
These are all parasites
done
clear
View Answer play_arrow
-
question_answer156) Which of the following statements regarding cilia is not correct?
A)
The organized beating of cilia is controlled by fluxes of \[C{{a}^{2+}}\] across the membrane
done
clear
B)
Cilia are hair-like cellular appendages
done
clear
C)
Microtubules of cilia are composed of tubulin
done
clear
D)
Cilia contain an outer ring of nine doublet microtubules surrounding two single microtubules
done
clear
View Answer play_arrow
-
question_answer157) Microbes found to be very useful in genetic engineering are:
A)
Escherichia coli and Agrobacterium tumefaciens
done
clear
B)
Vibrio choleras and a tailed bacteriophage
done
clear
C)
Diplococcus sp. and Pseudomonas sp.
done
clear
D)
Crown gall bacterium and Cacnorhabditis elegans
done
clear
View Answer play_arrow
-
question_answer158) Which of the following environmental conditions are essential for optimum growth of Mucor on a piece of bread?
A)
A. Temperature of about \[{{25}^{\text{o}}}C\] B. Temperature of about \[{{5}^{\text{o}}}C\] C. Relative humidity of about 5% D. Relative humidity of about 95% E. A shady place F. A brightly illuminated place Choose the answer from the following options: A, C and E only
done
clear
B)
A, D and E only
done
clear
C)
B, D and E only
done
clear
D)
B, C and F only
done
clear
View Answer play_arrow
-
question_answer159) Evolutionary history of an organism is known as:
A)
Phylogeny
done
clear
B)
Ancestry
done
clear
C)
Paleontology
done
clear
D)
Ontogeny
done
clear
View Answer play_arrow
-
question_answer160) Which of the following is considered a hot-spot of biodiversity in India?
A)
Western ghats
done
clear
B)
Indo-Gangetic plain
done
clear
C)
Eastern ghats
done
clear
D)
Aravalli hills
done
clear
View Answer play_arrow
-
question_answer161) During photorespiration, the oxygen consuming reaction (s) occur in:
A)
stroma of chloroplasts and mitochondria
done
clear
B)
stroma of chloroplasts and peroxisomes
done
clear
C)
grana of chloroplasts and peroxisomes
done
clear
D)
stroma of chloroplasts
done
clear
View Answer play_arrow
-
question_answer162) Which one of the following is an example of polygenic inheritance?
A)
Flower colour in Mirabilis jalapa
done
clear
B)
Production of male honey bee
done
clear
C)
Pod shape in garden pea
done
clear
D)
Skin colour in humans
done
clear
View Answer play_arrow
-
question_answer163) Which one of the following not act as a neurotransmitter?
A)
Acetylcholine
done
clear
B)
Epinephrine
done
clear
C)
Norepinephrine
done
clear
D)
Cortisone
done
clear
View Answer play_arrow
-
question_answer164) Sertoli cells are regulated by the pituitary hormone known as:
A)
FSH
done
clear
B)
GH
done
clear
C)
Prolactin
done
clear
D)
LH
done
clear
View Answer play_arrow
-
question_answer165) A steroid hormone which regulates glucose metabolism is:
A)
Cortisol
done
clear
B)
corticosterone
done
clear
C)
11 ?deoxycorticosterone
done
clear
D)
cortisone
done
clear
View Answer play_arrow
-
question_answer166) The contractile protein of skeletal muscle involving ATPase activity is:
A)
tropomyosin
done
clear
B)
myosin
done
clear
C)
a-actinin
done
clear
D)
troponin
done
clear
View Answer play_arrow
-
question_answer167) Which one of the following is not a second messenger in hormone action?
A)
cGMP
done
clear
B)
Calcium
done
clear
C)
Sodium
done
clear
D)
cAMP
done
clear
View Answer play_arrow
-
question_answer168) In Mendel's experiments with garden pea, round seed shape (RR) was dominant over wrinkled seeds (rr), yellow cotyledon CYY) was dominant over green cotyledon (yy). What are the expected phenotypes in the \[{{F}_{2}}\] generation of the cross \[RRYY\times rryy\]?
A)
Only round seeds with green cotyledons
done
clear
B)
Only wrinkled seeds with yellow cotyledons
done
clear
C)
Only wrinkled seeds with green cotyledons
done
clear
D)
Round seeds with yellow cotyledons and wrinkled seeds with yellow cotyledons
done
clear
View Answer play_arrow
-
question_answer169) One gene - one enzyme hypothesis was postulated by:
A)
R. Franklin
done
clear
B)
Hershey and Chase
done
clear
C)
A. Garrod
done
clear
D)
Beadle and Tatum
done
clear
View Answer play_arrow
-
question_answer170) One turn of the helix in a B-form DNA is approximately:
A)
20 nm
done
clear
B)
0.34 nm
done
clear
C)
3.4 nm
done
clear
D)
2 nm
done
clear
View Answer play_arrow
-
question_answer171) Test cross involves:
A)
crossing between two genotypes with recessive trait
done
clear
B)
crossing between two \[{{F}_{1}}\] hybrids
done
clear
C)
crossing the \[{{F}_{1}}\] hybrid with a double recessive genotype
done
clear
D)
crossing between two genotypes with dominant trait
done
clear
View Answer play_arrow
-
question_answer172) Antiparallel strands of a DNA molecule means that:
A)
one strand turns anti-clockwise
done
clear
B)
the phosphate groups of two DNA strands, at their ends, share the same position
done
clear
C)
the phosphate groups at the start of two DNA strands are in opposite position (pole)
done
clear
D)
one strand turns clockwise
done
clear
View Answer play_arrow
-
question_answer173) Areolar connective tissue joins:
A)
fat body with muscles
done
clear
B)
integument with muscles
done
clear
C)
bones with muscles
done
clear
D)
bones with bones
done
clear
View Answer play_arrow
-
question_answer174) Mast cells secrete:
A)
hippurin
done
clear
B)
myoglobin
done
clear
C)
histamine
done
clear
D)
haemoglobin Histamine is a protein, acting as a vasodilator (widening of blood vessels) in inflammatory and allergic reactions and also increases the permeability of small vessels. It is secreted from the mast cells which widely occur in the areolar connective tissue. Two other active substances secreted by mast cells are Heparin, a proteoglycan, which prevents coagulation of blood vessels and Serotonin, a protein, which acts as vasoconstrictor to stop bleeding and to increase blood pressure.
done
clear
View Answer play_arrow
-
question_answer175) If a colourblind woman marries a normal visioned man, their sons will be:
A)
all normal visioned
done
clear
B)
one-half colourblind and one-half normal
done
clear
C)
three-fourths coloiirbling and one-fourth normal
done
clear
D)
all colourblind
done
clear
View Answer play_arrow
-
question_answer176) Cri-du-chat syndrome in humans is caused by the:
A)
fertilization of an XX egg by a normal Y-bearing sperm
done
clear
B)
loss of half of the short arm of chromosome 5
done
clear
C)
loss of half of the long arm of chromosome 5
done
clear
D)
trisomy of 21st chromosome
done
clear
View Answer play_arrow
-
question_answer177) Restriction endonuclease:
A)
cuts the DNA molecule randomly
done
clear
B)
cuts the DNA molecule at specific sites
done
clear
C)
restricts the synthesis of DNA inside the nucleus
done
clear
D)
synthesizes DNA
done
clear
View Answer play_arrow
-
question_answer178) Antibodies in our body are complex:
A)
lipoproteins
done
clear
B)
steroids
done
clear
C)
prostaglandins
done
clear
D)
glycoproteins
done
clear
View Answer play_arrow
-
question_answer179) Limit of BOD prescribed by Central Pollution Control Board for the discharge of industrial and municipal waste water into natural surface water, is:
A)
< 3.0 ppm
done
clear
B)
< 10 ppm
done
clear
C)
< 100 ppm
done
clear
D)
< 30 ppm
done
clear
View Answer play_arrow
-
question_answer180) Earthworms are:
A)
ureotelic when, plenty of water is available
done
clear
B)
uricotelic when plenty of water is available
done
clear
C)
uricotelic under conditions of water scarcity
done
clear
D)
ammonotelic when plenty of water is available
done
clear
View Answer play_arrow
-
question_answer181) Which of the following is an accumulation and release centre of neurohormones?
A)
Posterior pituitary lobe
done
clear
B)
Intermediate lobe of the pituitary
done
clear
C)
Hypothalamus
done
clear
D)
Anterior pituitary lobe
done
clear
View Answer play_arrow
-
question_answer182) Withdrawal of which of the following hormones is the immediate cause of menstruation?
A)
Eastrogens
done
clear
B)
FSH
done
clear
C)
FSH-RH
done
clear
D)
Progesterone
done
clear
View Answer play_arrow
-
question_answer183) Which one of the following statements is incorrect?
A)
The residual air in lungs slightly decreases the efficiency of respiration in mammals
done
clear
B)
The presence of non-respiratory air sacs, increases the efficiency of respiration in birds
done
clear
C)
In insects, circulating body fluids serve to distribute oxygen to tissues
done
clear
D)
The principle of countercurrent flow facilitates efficient respiration in gills of fishes
done
clear
View Answer play_arrow
-
question_answer184) Which one of the following has an open circulatory system?
A)
Pheretima
done
clear
B)
Periplaneta
done
clear
C)
Hirudinaria
done
clear
D)
Octopus
done
clear
View Answer play_arrow
-
question_answer185) Which hormone causes dilation of blood vessels, increased oxygen consumption and glycogenotysis?
A)
ACTH
done
clear
B)
Insulin
done
clear
C)
Adrenalin
done
clear
D)
Glucagon
done
clear
View Answer play_arrow
-
question_answer186) The causative agent of mad-cow disease is a:
A)
bacterium
done
clear
B)
prion
done
clear
C)
worm
done
clear
D)
virus
done
clear
View Answer play_arrow
-
question_answer187) The translocation of organic solutes in sieve tube members is supported by:
A)
root pressure and transpiration pull
done
clear
B)
P-proteins
done
clear
C)
mass flow involving a carrier and ATP
done
clear
D)
cytoplasmic streaming
done
clear
View Answer play_arrow
-
question_answer188) Biradial symmetry and lack of cnidoblasts are the characteristics of:
A)
Starfish and sea anemone
done
clear
B)
Ctenoplana and Beroe
done
clear
C)
Aurelia and Paramecium
done
clear
D)
Hydra and starfish
done
clear
View Answer play_arrow
-
question_answer189) The arrangement of the nuclei in a normal embryo sac in the dicot plants is:
A)
2 + 4 + 2
done
clear
B)
3 + 2 + 3
done
clear
C)
2 + 3 + 3
done
clear
D)
3 + 3 + 2
done
clear
View Answer play_arrow
-
question_answer190) An enzyme that can stimulate germination of barley seeds is:
A)
\[\alpha \]-amylase
done
clear
B)
lipase
done
clear
C)
protease
done
clear
D)
invertase
done
clear
View Answer play_arrow
-
question_answer191) In a cereal grain the single cotyledon of embryo is represented by:
A)
coleorhiza
done
clear
B)
scutellum
done
clear
C)
prophyll
done
clear
D)
coleoptiles
done
clear
View Answer play_arrow
-
question_answer192) The majority of carbon dioxide produced by our body cells is transported to the lungs:
A)
dissolved in the blood
done
clear
B)
as bircarbonates
done
clear
C)
as carbonates
done
clear
D)
attached to haemoglobin
done
clear
View Answer play_arrow
-
question_answer193) Triticale, the first man-made cereal crop, has been obtained by crossing wheat with:
A)
rye
done
clear
B)
pearl millet
done
clear
C)
sugarcane
done
clear
D)
barley
done
clear
View Answer play_arrow
-
question_answer194) In order to obtain virus-free plants through tissue culture the best method is:
A)
protoplast culture
done
clear
B)
embryo rescue
done
clear
C)
anther culture
done
clear
D)
meristem culture
done
clear
View Answer play_arrow
-
question_answer195) HIV that causes AIDS, first starts destroying:
A)
B -lymph oocytes
done
clear
B)
leucocytes
done
clear
C)
thrombocytes
done
clear
D)
helper T-lymphocytes
done
clear
View Answer play_arrow
-
question_answer196) In which one of the following sets of animals do all the four give birth to young ones?
A)
Lion, bat, whale, ostrich
done
clear
B)
Platypus, penguin, bat, hippopotamus
done
clear
C)
Shrew, bat, cat, kiwi
done
clear
D)
Kangaroo, hedgehog, dolphin, loris
done
clear
View Answer play_arrow
-
question_answer197) Sickle cell anaemia has not been eliminated from the African population because:
A)
it is controlled by recessive genes
done
clear
B)
it is not a fatal disease
done
clear
C)
it provides immunity against malaria
done
clear
D)
it is controlled by dominant genes
done
clear
View Answer play_arrow
-
question_answer198) Two common characters found in centipede, cockroach and crab are:
A)
compound eyes and anal cerci
done
clear
B)
jointed legs and chitinous exoskeleton
done
clear
C)
green gland and tracheae
done
clear
D)
book lungs and antennae
done
clear
View Answer play_arrow
-
question_answer199) Both sickle cell anaemia and Huntington's chorea are:
A)
bacteria-related diseases
done
clear
B)
congenital disorders
done
clear
C)
pollutant-induced disorders
done
clear
D)
virus-related diseases
done
clear
View Answer play_arrow
-
question_answer200) Angiotensinogen is a protein produced and secreted by:
A)
macula densa cells
done
clear
B)
endothelial cells (cells lining the blood vessels)
done
clear
C)
liver cells
done
clear
D)
juxtaglomerular (JG) cells
done
clear
View Answer play_arrow

 The logic circuit gate is:
The logic circuit gate is:




 \[N{{i}^{2+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{{}}}\] \[_{22}Ti=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{2}},4{{s}^{2}}\]
\[N{{i}^{2+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{{}}}\] \[_{22}Ti=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{2}},4{{s}^{2}}\]  \[T{{i}^{3+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{1}}\] \[_{21}Sc=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{1}},4{{s}^{2}}\] \[S{{c}^{3+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}\] (unpaired electron in d-orbital is not possible) \[_{29}Cu=1{{s}^{2}},2{{s}^{2}}\,2{{p}^{6}},3{{s}^{2}}\,3{{p}^{6}}\,3{{d}^{10}},4{{s}^{1}}\] \[C{{u}^{+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}\] (complete d-orbital) Hence, in above ions, \[N{{i}^{2+}}\] and \[T{{i}^{3+}}\] ions are coloured ions in aqueous solution due to presence of unpaired electrons in d-sub-shell.
\[T{{i}^{3+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{1}}\] \[_{21}Sc=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{1}},4{{s}^{2}}\] \[S{{c}^{3+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}\] (unpaired electron in d-orbital is not possible) \[_{29}Cu=1{{s}^{2}},2{{s}^{2}}\,2{{p}^{6}},3{{s}^{2}}\,3{{p}^{6}}\,3{{d}^{10}},4{{s}^{1}}\] \[C{{u}^{+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}\] (complete d-orbital) Hence, in above ions, \[N{{i}^{2+}}\] and \[T{{i}^{3+}}\] ions are coloured ions in aqueous solution due to presence of unpaired electrons in d-sub-shell.  is:
is:
