A) linkage isomerism, ionization isomerism and optical isomerism
B) linkage isomerism, ionization isomerism and geometrical isomerism
C) ionization isomerism, geometrical isomerism and optical isomerism
D) linkage isomerism, geometrical isomerism and optical isomerism
Correct Answer: B
Solution :
The compound \[[Co{{(N{{H}_{3}})}_{4}}{{(N{{O}_{2}})}_{2}}\,]Cl\] exhibits linkage, ionization and geometrical isomerism. Hence, its linkage isomers are (i) \[[Co{{(N{{H}_{3}})}_{4}}{{(N{{O}_{2}})}_{2}}]Cl\] and \[[Co{{(N{{H}_{3}})}_{4}}{{(ONO)}_{2}}]Cl\] (ii) its ionisation isomers are \[[Co{{(N{{H}_{3}})}_{4}}(N{{O}_{2}})\,Cl]\,N{{O}_{2}}\] and \[[Co{{(N{{H}_{3}})}_{4}}{{(N{{O}_{2}})}_{2}}]Cl\] (iii) its geometrical isomers are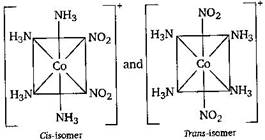
You need to login to perform this action.
You will be redirected in
3 sec
