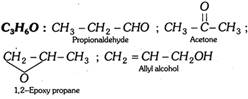
 (iii) Acids, esters and hydroxy carbonyl compounds …etc. (Cn H2nO2)
C2H4O2 : \[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,\] ; \[\underset{\text{Methyl formate}}{\mathop{HCOOC{{H}_{3}}}}\,\]
C3H6O2 : \[\underset{\text{Propionic acid}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-COOH}}\,\] ; \[\underset{\text{Methyl acetate}}{\mathop{C{{H}_{3}}COOC{{H}_{3}}}}\,\] ;
\[\underset{2-\,\text{Hydroxy propanal}}{\mathop{C{{H}_{3}}\underset{OH\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,HCHO}}\,}}\,\]; \[\underset{1-\,\text{Hydroxy propan-2-one}}{\mathop{C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{2}}-OH}}\,\]
(iv) Alkynes and alkadienes (Cn H2n-2)
C4H6 : \[\underset{1-\,\text{Butyne}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C\equiv CH}}\,\]; \[\underset{1,3-\,\text{Butadiene}}{\mathop{{{H}_{2}}C=CH-CH=C{{H}_{2}}}}\,\];
\[\underset{2-\text{Butyne}}{\mathop{C{{H}_{3}}-C\equiv C-C{{H}_{3}}}}\,\] ; \[\underset{1,2-\text{ Butadiene}}{\mathop{{{H}_{2}}C=C=CH-C{{H}_{3}}}}\,\]
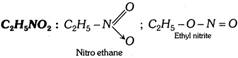
(v) Nitro alkanes and alkyl nitrites (\[-N{{O}_{2}}\]and \[-O-N=O\])
(iii) Acids, esters and hydroxy carbonyl compounds …etc. (Cn H2nO2)
C2H4O2 : \[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,\] ; \[\underset{\text{Methyl formate}}{\mathop{HCOOC{{H}_{3}}}}\,\]
C3H6O2 : \[\underset{\text{Propionic acid}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-COOH}}\,\] ; \[\underset{\text{Methyl acetate}}{\mathop{C{{H}_{3}}COOC{{H}_{3}}}}\,\] ;
\[\underset{2-\,\text{Hydroxy propanal}}{\mathop{C{{H}_{3}}\underset{OH\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,HCHO}}\,}}\,\]; \[\underset{1-\,\text{Hydroxy propan-2-one}}{\mathop{C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{2}}-OH}}\,\]
(iv) Alkynes and alkadienes (Cn H2n-2)
C4H6 : \[\underset{1-\,\text{Butyne}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C\equiv CH}}\,\]; \[\underset{1,3-\,\text{Butadiene}}{\mathop{{{H}_{2}}C=CH-CH=C{{H}_{2}}}}\,\];
\[\underset{2-\text{Butyne}}{\mathop{C{{H}_{3}}-C\equiv C-C{{H}_{3}}}}\,\] ; \[\underset{1,2-\text{ Butadiene}}{\mathop{{{H}_{2}}C=C=CH-C{{H}_{3}}}}\,\]
(v) Nitro alkanes and alkyl nitrites (\[-N{{O}_{2}}\]and \[-O-N=O\])
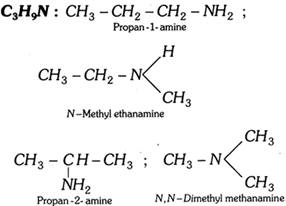
 (vi) Amines (Primary, secondary and tertiary)
(vi) Amines (Primary, secondary and tertiary)
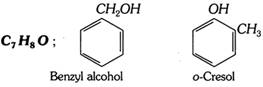
 (vii) Alcohols and phenols
(vii) Alcohols and phenols
 (viii) Oximes and amides
C2H5NO : \[\underset{\text{Acetaldoxime}}{\mathop{C{{H}_{3}}-CH=NOH}}\,\]; \[\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}\overset{O}{\mathop{-\overset{||}{\mathop{C}}\,-}}\,N{{H}_{2}}}}\,\]
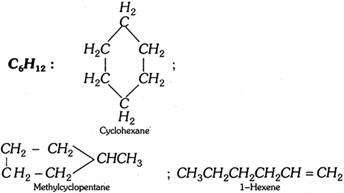
(4) Ring-chain isomerism : This type of isomerism is due to different modes of linking of carbon atoms, i.e., the isomers possess either open chain or closed chain sturctures.
(viii) Oximes and amides
C2H5NO : \[\underset{\text{Acetaldoxime}}{\mathop{C{{H}_{3}}-CH=NOH}}\,\]; \[\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}\overset{O}{\mathop{-\overset{||}{\mathop{C}}\,-}}\,N{{H}_{2}}}}\,\]
(4) Ring-chain isomerism : This type of isomerism is due to different modes of linking of carbon atoms, i.e., the isomers possess either open chain or closed chain sturctures.
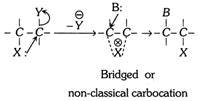 X = Nucleophilic species, Y = Electronegative group, B = Another nucleophile.
(2) Rearrangement or migration to electron rich atoms (Electrophilic rearrangement) : Those rearrangement reactions in which migrating group is electrophile and thus migrates to electron rich centre.
(3) Rearrangement or migration to free radical species (Free radical rearrangement) : Those rearrangement reactions in which the migrating group moves to a free radical centre. Free radical rearrangements are comparatively rare.
(4) Aromatic rearrangement : Those rearrangement reactions in which the migrating group moves to aromatic nucleus. Aromatic compounds of the type (I) undergo rearrangements in the manner mentioned below,
X = Nucleophilic species, Y = Electronegative group, B = Another nucleophile.
(2) Rearrangement or migration to electron rich atoms (Electrophilic rearrangement) : Those rearrangement reactions in which migrating group is electrophile and thus migrates to electron rich centre.
(3) Rearrangement or migration to free radical species (Free radical rearrangement) : Those rearrangement reactions in which the migrating group moves to a free radical centre. Free radical rearrangements are comparatively rare.
(4) Aromatic rearrangement : Those rearrangement reactions in which the migrating group moves to aromatic nucleus. Aromatic compounds of the type (I) undergo rearrangements in the manner mentioned below,
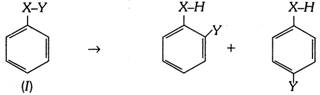 The element X from which group Y migrates may be nitrogen or oxygen.
The element X from which group Y migrates may be nitrogen or oxygen. 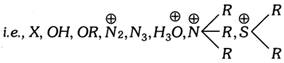 Elimination reactions are generally endothermic and take place on heating.
Elimination reactions are classified into two general types,
(I) \[\alpha -\]elimination reactions or 1, 1-elimination reactions.
(II) \[\beta -\]elimination reaction or 1, 2-elimination reactions.
(I) \[\alpha -\]elimination reactions or 1,1-elimination reactions: A reaction in which both the groups or atoms are removed from the same carbon of the molecule is called \[\alpha -\]elimination reaction. This reaction is mainly given by gem dihalides and gem trihalides having at least one \[\alpha -\]hydrogen.
\[CH{{X}_{3}}\xrightarrow{\text{Alc}\text{.}\,\,KOH\text{/}\Delta }\overset{.\,.\,\,\,\,}{\mathop{C{{X}_{2}}}}\,+\overset{\Theta }{\mathop{X}}\,+\overset{\oplus }{\mathop{H}}\,\]
Product of the reaction is halocarbenes or dihalocarbenes. which are key intermediates in a wide variety of chemical and photochemical reactions.
(II) \[\beta -\]elimination reactions or 1, 2-elimination reactions: Consider the following reactions,
\[C{{H}_{3}}-\underset{\beta }{\mathop{C{{H}_{2}}}}\,-\underset{\alpha }{\mathop{C{{H}_{2}}}}\,-L\to C{{H}_{3}}-CH=C{{H}_{2}}+\overset{\oplus }{\mathop{H}}\,+\overset{\Theta }{\mathop{L}}\,\]
A reaction in which functional group (i.e., leaving group) is removed from \[\alpha -\]carbon and other group (Generally hydrogen atom) from the \[\beta -\]carbon is called \[\beta -\]elimination reaction. In this reaction there is loss of two \[\sigma \] bonds and gain of one \[\pi \] bond. Product of the reaction is generally less stable than the reactant.
(1) Types of \[\beta -\]elimination reactions : In analogy with substitution reactions, \[\beta -\] elimination reactions are divided into three types:
(i) \[{{E}_{1}}\] (Elimination unimolecular) reaction, (ii) \[{{E}_{2}}\] (Elimination bimolecular) reaction and (iii) \[{{E}_{1\,cb}}\] (Elimination unimolecular conjugate base) reaction
(i) \[{{E}_{1}}\] (Elimination unimolecular) reaction : Consider the following reaction,
Elimination reactions are generally endothermic and take place on heating.
Elimination reactions are classified into two general types,
(I) \[\alpha -\]elimination reactions or 1, 1-elimination reactions.
(II) \[\beta -\]elimination reaction or 1, 2-elimination reactions.
(I) \[\alpha -\]elimination reactions or 1,1-elimination reactions: A reaction in which both the groups or atoms are removed from the same carbon of the molecule is called \[\alpha -\]elimination reaction. This reaction is mainly given by gem dihalides and gem trihalides having at least one \[\alpha -\]hydrogen.
\[CH{{X}_{3}}\xrightarrow{\text{Alc}\text{.}\,\,KOH\text{/}\Delta }\overset{.\,.\,\,\,\,}{\mathop{C{{X}_{2}}}}\,+\overset{\Theta }{\mathop{X}}\,+\overset{\oplus }{\mathop{H}}\,\]
Product of the reaction is halocarbenes or dihalocarbenes. which are key intermediates in a wide variety of chemical and photochemical reactions.
(II) \[\beta -\]elimination reactions or 1, 2-elimination reactions: Consider the following reactions,
\[C{{H}_{3}}-\underset{\beta }{\mathop{C{{H}_{2}}}}\,-\underset{\alpha }{\mathop{C{{H}_{2}}}}\,-L\to C{{H}_{3}}-CH=C{{H}_{2}}+\overset{\oplus }{\mathop{H}}\,+\overset{\Theta }{\mathop{L}}\,\]
A reaction in which functional group (i.e., leaving group) is removed from \[\alpha -\]carbon and other group (Generally hydrogen atom) from the \[\beta -\]carbon is called \[\beta -\]elimination reaction. In this reaction there is loss of two \[\sigma \] bonds and gain of one \[\pi \] bond. Product of the reaction is generally less stable than the reactant.
(1) Types of \[\beta -\]elimination reactions : In analogy with substitution reactions, \[\beta -\] elimination reactions are divided into three types:
(i) \[{{E}_{1}}\] (Elimination unimolecular) reaction, (ii) \[{{E}_{2}}\] (Elimination bimolecular) reaction and (iii) \[{{E}_{1\,cb}}\] (Elimination unimolecular conjugate base) reaction
(i) \[{{E}_{1}}\] (Elimination unimolecular) reaction : Consider the following reaction,
 (a) Reaction velocity depends only on the concentration of the substrate; thus reaction is unimolecular reaction.
Rate \[\propto \] [Substrate]
(b) Product formation takes place by the formation of carbocation as reaction intermediate \[(RI)\].
(c) Since reaction intermediate is carbocation, rearrangement is possible in \[{{E}_{1}}\] reaction.
(d) Reaction is carried out in the presence of polar protic solvent.
(e) The \[{{E}_{1}}\] reaction occurs in two steps,
Step 1.
(a) Reaction velocity depends only on the concentration of the substrate; thus reaction is unimolecular reaction.
Rate \[\propto \] [Substrate]
(b) Product formation takes place by the formation of carbocation as reaction intermediate \[(RI)\].
(c) Since reaction intermediate is carbocation, rearrangement is possible in \[{{E}_{1}}\] reaction.
(d) Reaction is carried out in the presence of polar protic solvent.
(e) The \[{{E}_{1}}\] reaction occurs in two steps,
Step 1.
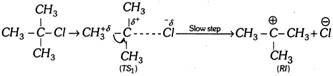 Step 2.
Step 2.
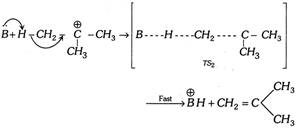 (ii) \[{{E}_{2}}\] (Elimination bimolecular) reaction : Consider the following reaction,
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-Br\underset{\Delta }{\mathop{\xrightarrow{Base\,(B)}}}\,C{{H}_{3}}-CH=C{{H}_{2}}+\overset{\oplus }{\mathop{H}}\,+\overset{\Theta }{\mathop{Br}}\,\]
(a) Reaction velocity depends only on the concentration of the substrate and the base used; thus reaction is bimolecular reaction. Rate \[\propto \][Substrate] [Base]
(b) Since the reaction is a bimolecular reaction, the product formation will take place by formation of transition state (TS).
(c) Rearrangement does not take place in \[{{E}_{2}}\] reaction but in case of allylic compound rearrangement is possible.
(d) Reaction is carried out in the presence of polar aprotic solvent.
(e) The \[{{E}_{2}}\] reaction occurs in one step,
(ii) \[{{E}_{2}}\] (Elimination bimolecular) reaction : Consider the following reaction,
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-Br\underset{\Delta }{\mathop{\xrightarrow{Base\,(B)}}}\,C{{H}_{3}}-CH=C{{H}_{2}}+\overset{\oplus }{\mathop{H}}\,+\overset{\Theta }{\mathop{Br}}\,\]
(a) Reaction velocity depends only on the concentration of the substrate and the base used; thus reaction is bimolecular reaction. Rate \[\propto \][Substrate] [Base]
(b) Since the reaction is a bimolecular reaction, the product formation will take place by formation of transition state (TS).
(c) Rearrangement does not take place in \[{{E}_{2}}\] reaction but in case of allylic compound rearrangement is possible.
(d) Reaction is carried out in the presence of polar aprotic solvent.
(e) The \[{{E}_{2}}\] reaction occurs in one step,
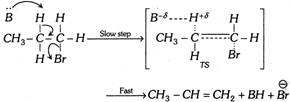 more...
more... 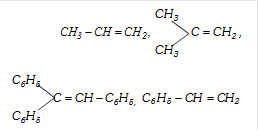 Following alkenes will not give addition reaction according to Markownikoff's rule
Following alkenes will not give addition reaction according to Markownikoff's rule
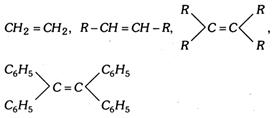 (vi) Unsymmetrical alkenes having the following general structure give addition according to anti Markownikoff's rule. \[C{{H}_{2}}=CH-G\], where G is a strong - I group such as
\[-C{{X}_{3}},-N{{O}_{2,}}-CN,-CHO,-COR,-COOH,-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-Z\,\,\]\[(Z=Cl,OH,OR,N{{H}_{2}})\]
Example:
\[C{{H}_{2}}=CH-CHO+HCl\xrightarrow{\text{Anti-Markownikoff }\!\!'\!\!\text{ s addition}}{{\overset{Cl\,}{\mathop{\overset{|\,\,\,\,\,\,}{\mathop{CH}}\,}}\,}_{2}}-C{{H}_{2}}-CHO\]
(vii) Mechanism of electrophilic addition reactions is as follows,
(vi) Unsymmetrical alkenes having the following general structure give addition according to anti Markownikoff's rule. \[C{{H}_{2}}=CH-G\], where G is a strong - I group such as
\[-C{{X}_{3}},-N{{O}_{2,}}-CN,-CHO,-COR,-COOH,-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-Z\,\,\]\[(Z=Cl,OH,OR,N{{H}_{2}})\]
Example:
\[C{{H}_{2}}=CH-CHO+HCl\xrightarrow{\text{Anti-Markownikoff }\!\!'\!\!\text{ s addition}}{{\overset{Cl\,}{\mathop{\overset{|\,\,\,\,\,\,}{\mathop{CH}}\,}}\,}_{2}}-C{{H}_{2}}-CHO\]
(vii) Mechanism of electrophilic addition reactions is as follows,
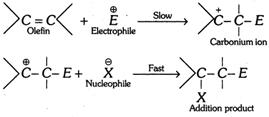 (2) Nucleophilic addition reactions : When the addition reaction occurs on account of the initial attack of nucleophile, the reaction is said to be a nucleophilic addition reaction. Due to presence of strongly electronegative oxygen atom, the \[\pi -\]electrons of the carbon-oxygen double bond in carbonyl group (\[C=O\]) get shifted towards the oxygen atom and thereby such bond is highly polarised. This makes carbon atom of the carbonyl group electron deficient.
(2) Nucleophilic addition reactions : When the addition reaction occurs on account of the initial attack of nucleophile, the reaction is said to be a nucleophilic addition reaction. Due to presence of strongly electronegative oxygen atom, the \[\pi -\]electrons of the carbon-oxygen double bond in carbonyl group (\[C=O\]) get shifted towards the oxygen atom and thereby such bond is highly polarised. This makes carbon atom of the carbonyl group electron deficient.
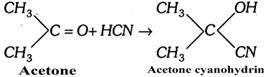 The mechanism of the reaction involves the following steps:
Step 1. HCN gives a proton \[(\overset{\oplus }{\mathop{H}}\,)\] and a nucleophile, cyanide ion \[(\overset{\Theta }{\mathop{CN}}\,)\].
\[HCN\to {{H}^{\oplus }}+C{{N}^{\Theta }}\]
Step 2. The nucleophile \[(C{{N}^{\Theta }})\] attacks the positively charged carbon so as to form an anion [\[{{H}^{\oplus }}\] does more...
The mechanism of the reaction involves the following steps:
Step 1. HCN gives a proton \[(\overset{\oplus }{\mathop{H}}\,)\] and a nucleophile, cyanide ion \[(\overset{\Theta }{\mathop{CN}}\,)\].
\[HCN\to {{H}^{\oplus }}+C{{N}^{\Theta }}\]
Step 2. The nucleophile \[(C{{N}^{\Theta }})\] attacks the positively charged carbon so as to form an anion [\[{{H}^{\oplus }}\] does more... 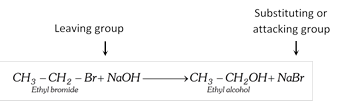 (Bromine atom is replaced by hydroxyl group)
Types of substitution reactions : On the basis of the nature of attacking species substitution reactions are classified into following three categories,
(1) Nucleophilic substitution reactions
(2) Electrophilic substitution reactions
(3) Free radical substitution reactions
(1) Nucleophilic substitution reactions
(i) Many substitution reactions, especially at the saturated carbon atom in aliphatic compounds such as alkyl halides, are brought about by nucleophilic reagents or nucleophiles.
\[\underset{Substrate}{\mathop{R-X}}\,+\underset{Nucleophile}{\mathop{O{{H}^{\Theta }}}}\,\xrightarrow{\,\,\,\,\,\,\,\,\,}R-OH+\underset{Leaving\,group}{\mathop{{{X}^{\Theta }}}}\,\]
Such substitution reactions are called nucleophilic substitution reactions, i.e., \[{{S}_{N}}\]reactions (S stands for substitution and N for nucleophile).
(ii) The weaker the basicity of a group of the substrate, the better is its leaving ability.
Leaving power of the group \[\propto \frac{1}{\text{Basicity}\,\text{of}\,\text{the}\,\text{group}}\]
Example :
\[\underset{\text{Decreasing acidity}}{\mathop{\xrightarrow{HI>HBr>HCl>HF}}}\,\]
\[\underset{\underset{\underset{\text{Decreasing}\,\text{leaving}\,\text{ability}}{\mathop{\Downarrow }}\,}{\mathop{\text{Increasing basicity}}}\,}{\mathop{\xrightarrow{\,\,\,\,\,\overset{\Theta }{\mathop{I}}\,\,\overset{\Theta }{\mathop{\,\,\,\,\,Br\,\,\,\,\,\,}}\,\overset{\Theta }{\mathop{Cl}}\,\,\,\,\,\,\,\overset{\Theta }{\mathop{F}}\,\,\,\,\,\,\,\,}}}\,\]
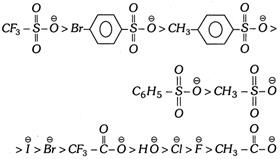
(iii) The leaving power of some nucleophilic groups are given below in decreasing order,
(Bromine atom is replaced by hydroxyl group)
Types of substitution reactions : On the basis of the nature of attacking species substitution reactions are classified into following three categories,
(1) Nucleophilic substitution reactions
(2) Electrophilic substitution reactions
(3) Free radical substitution reactions
(1) Nucleophilic substitution reactions
(i) Many substitution reactions, especially at the saturated carbon atom in aliphatic compounds such as alkyl halides, are brought about by nucleophilic reagents or nucleophiles.
\[\underset{Substrate}{\mathop{R-X}}\,+\underset{Nucleophile}{\mathop{O{{H}^{\Theta }}}}\,\xrightarrow{\,\,\,\,\,\,\,\,\,}R-OH+\underset{Leaving\,group}{\mathop{{{X}^{\Theta }}}}\,\]
Such substitution reactions are called nucleophilic substitution reactions, i.e., \[{{S}_{N}}\]reactions (S stands for substitution and N for nucleophile).
(ii) The weaker the basicity of a group of the substrate, the better is its leaving ability.
Leaving power of the group \[\propto \frac{1}{\text{Basicity}\,\text{of}\,\text{the}\,\text{group}}\]
Example :
\[\underset{\text{Decreasing acidity}}{\mathop{\xrightarrow{HI>HBr>HCl>HF}}}\,\]
\[\underset{\underset{\underset{\text{Decreasing}\,\text{leaving}\,\text{ability}}{\mathop{\Downarrow }}\,}{\mathop{\text{Increasing basicity}}}\,}{\mathop{\xrightarrow{\,\,\,\,\,\overset{\Theta }{\mathop{I}}\,\,\overset{\Theta }{\mathop{\,\,\,\,\,Br\,\,\,\,\,\,}}\,\overset{\Theta }{\mathop{Cl}}\,\,\,\,\,\,\,\overset{\Theta }{\mathop{F}}\,\,\,\,\,\,\,\,}}}\,\]
(iii) The leaving power of some nucleophilic groups are given below in decreasing order,
 (iv) In these reactions leaving group of the substrate is replaced by another nucleophile. If reagent is neutral then leaving group is replaced by negative part of the reagent. Negative part of the reagent is always nucleophilic in character.
\[R-L\xrightarrow{\overset{+\delta }{\mathop{E}}\,-\overset{-\delta }{\mathop{Nu}}\,}R-Nu+\overset{\Theta }{\mathop{L}}\,\]; \[R-L+\overset{\Theta }{\mathop{Nu}}\,\xrightarrow{{}}R-Nu+\overset{\Theta }{\mathop{L}}\,\]
(v) In \[{{S}_{N}}\] reactions basicity of leaving group should be less than the basicity of incoming nucleophilic group. Thus strongly basic nucleophilic group replaces weakly basic nucleophilic group of the substrate.
Example :
\[R-Cl\underset{(NaOH)}{\mathop{\xrightarrow{O{{H}^{\Theta }}}}}\,R-OH+\overset{\Theta }{\mathop{Cl}}\,\] .....(A)
Basicity of \[O{{H}^{\Theta }}\]is more than\[\overset{\Theta }{\mathop{Cl}}\,\]hence\[\overset{\Theta }{\mathop{OH}}\,\]replaces Cl as \[\overset{\Theta }{\mathop{Cl}}\,\].
\[R-OH\underset{(HCl)}{\mathop{\xrightarrow{\overset{\Theta }{\mathop{Cl}}\,\,}}}\,R-Cl+\overset{\Theta }{\mathop{OH}}\,\] ......(B)
Basicity of \[\overset{\Theta }{\mathop{Cl}}\,\] is less than \[\overset{\Theta }{\mathop{OH}}\,\], hence \[\overset{\Theta }{\mathop{Cl}}\,\] will not replace OH as \[\overset{\Theta }{\mathop{OH}}\,\] hence reaction (B) will not occur.
(vi) Unlike aliphatic compounds having nucleophilic group as leaving group, aromatic compounds having same group bonded directly with aromatic ring do not undergo nucleophilic substitution reaction under ordinary conditions.
The reason for this unusual reactivity is the presence of lone pair of electron or \[\pi \] bond on the key atom of the functional group. Another factor for the low reactivity is nucleophilic character of aromatic ring.
(vii) The \[{{S}_{N}}\] reactions are divided into two classes, \[{{S}_{{{N}^{2}}}}\] and \[{{S}_{{{N}^{1}}}}\] reactions.
Distinction between \[{{S}_{{{N}^{2}}}}\] and \[{{S}_{{{N}^{1}}}}\] reactions
(iv) In these reactions leaving group of the substrate is replaced by another nucleophile. If reagent is neutral then leaving group is replaced by negative part of the reagent. Negative part of the reagent is always nucleophilic in character.
\[R-L\xrightarrow{\overset{+\delta }{\mathop{E}}\,-\overset{-\delta }{\mathop{Nu}}\,}R-Nu+\overset{\Theta }{\mathop{L}}\,\]; \[R-L+\overset{\Theta }{\mathop{Nu}}\,\xrightarrow{{}}R-Nu+\overset{\Theta }{\mathop{L}}\,\]
(v) In \[{{S}_{N}}\] reactions basicity of leaving group should be less than the basicity of incoming nucleophilic group. Thus strongly basic nucleophilic group replaces weakly basic nucleophilic group of the substrate.
Example :
\[R-Cl\underset{(NaOH)}{\mathop{\xrightarrow{O{{H}^{\Theta }}}}}\,R-OH+\overset{\Theta }{\mathop{Cl}}\,\] .....(A)
Basicity of \[O{{H}^{\Theta }}\]is more than\[\overset{\Theta }{\mathop{Cl}}\,\]hence\[\overset{\Theta }{\mathop{OH}}\,\]replaces Cl as \[\overset{\Theta }{\mathop{Cl}}\,\].
\[R-OH\underset{(HCl)}{\mathop{\xrightarrow{\overset{\Theta }{\mathop{Cl}}\,\,}}}\,R-Cl+\overset{\Theta }{\mathop{OH}}\,\] ......(B)
Basicity of \[\overset{\Theta }{\mathop{Cl}}\,\] is less than \[\overset{\Theta }{\mathop{OH}}\,\], hence \[\overset{\Theta }{\mathop{Cl}}\,\] will not replace OH as \[\overset{\Theta }{\mathop{OH}}\,\] hence reaction (B) will not occur.
(vi) Unlike aliphatic compounds having nucleophilic group as leaving group, aromatic compounds having same group bonded directly with aromatic ring do not undergo nucleophilic substitution reaction under ordinary conditions.
The reason for this unusual reactivity is the presence of lone pair of electron or \[\pi \] bond on the key atom of the functional group. Another factor for the low reactivity is nucleophilic character of aromatic ring.
(vii) The \[{{S}_{N}}\] reactions are divided into two classes, \[{{S}_{{{N}^{2}}}}\] and \[{{S}_{{{N}^{1}}}}\] reactions.
Distinction between \[{{S}_{{{N}^{2}}}}\] and \[{{S}_{{{N}^{1}}}}\] reactions
| Factors | more...
The fission of the substrate molecule to create centres of high or low electron density is influenced by attacking reagents. Most of the attacking reagents can be classified into two main groups.
Electrophiles or electrophilic reagents and Nucleophiles or nucleophilic reagents.
(1) Electrophiles : Electron deficient species or electron acceptor is an electrophile.
It can be classified into two categories :
(i) Charged electrophiles : Positively charged species in which central atom has incomplete octet is called charged electrophile.
Short lived fragments called reaction intermediates result from homolytic and heterolytic bond fission. The important reaction intermediates are free radicals, carbocations, carbanions, carbenes, benzyne and nitrenes.
Negativecharge on C
|