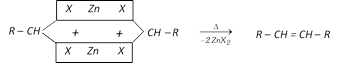
 (iv) By action of on vicinal dihalide :
(iv) By action of on vicinal dihalide :
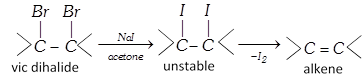 (v) From alcohols [Laboratory method] :
\[\underset{\text{Ethyl alcohol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\underset{443\,K}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}\,or\,{{H}_{3}}P{{O}_{4}}}}}\,\underset{\text{Ethene}}{\mathop{C{{H}_{2}}}}\,=C{{H}_{2}}+{{H}_{2}}O\]
(vi) Kolbe’s reaction :
\[\underset{\text{Potassium succinate}}{\mathop{\begin{array}{*{35}{l}} C{{H}_{2}}COOK \\ \,| \\ C{{H}_{2}}COOK \\ \end{array}}}\,+2{{H}_{2}}O\xrightarrow{\text{Electrolysis}}\underset{\text{Ethene}}{\mathop{\begin{array}{*{35}{l}} C{{H}_{2}} \\ \,| \\ C{{H}_{2}} \\ \end{array}}}\,+2C{{O}_{2}}+{{H}_{2}}+2KOH\]
(vii) From esters [Pyrolysis of ester] :
\[C{{H}_{3}}-CO-\underset{C{{H}_{2}}-C{{H}_{2}}}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|\,\,\,\,\,\,\,}{\mathop{O\,\,\,\,\,\,\,\,\,\,\,\,H\,\,\,\,\,\,}}\,}}\,\underset{liq.\,{{N}_{2}}}{\mathop{\begin{align} & \\ & \xrightarrow{\text{Glass}\,\text{wool}\,{{450}^{o}}} \\ \end{align}}}\,\underset{C{{H}_{2}}=C{{H}_{2}}}{\mathop{\underset{+}{\mathop{C{{H}_{3}}-COOH}}\,}}\,\]
(viii) Pyrolysis of quaternary ammonium compounds :
\[\underset{\underset{\text{hydroxide}}{\mathop{\text{Tetraethyl}\,\text{ammonium}}}\,}{\mathop{{{({{C}_{2}}{{H}_{5}})}_{4}}\overset{+}{\mathop{N}}\,\overset{-}{\mathop{OH}}\,}}\,\xrightarrow{heat}\underset{\underset{\text{(Tert}\text{.}\,\text{amine)}}{\mathop{\text{Triethylamine}}}\,}{\mathop{{{({{C}_{2}}{{H}_{5}})}_{3}}N}}\,+\underset{\text{Ethene}}{\mathop{{{C}_{2}}{{H}_{4}}}}\,+{{H}_{2}}O\]
(ix) Action of copper alkyl on vinyl chloride :
\[\underset{\text{Vinyl}\,\text{chloride}}{\mathop{{{H}_{2}}C=CHCl}}\,\xrightarrow{Cu{{R}_{2}}}{{H}_{2}}C=CHR\]
(x) By Grignard reagents :
(v) From alcohols [Laboratory method] :
\[\underset{\text{Ethyl alcohol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\underset{443\,K}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}\,or\,{{H}_{3}}P{{O}_{4}}}}}\,\underset{\text{Ethene}}{\mathop{C{{H}_{2}}}}\,=C{{H}_{2}}+{{H}_{2}}O\]
(vi) Kolbe’s reaction :
\[\underset{\text{Potassium succinate}}{\mathop{\begin{array}{*{35}{l}} C{{H}_{2}}COOK \\ \,| \\ C{{H}_{2}}COOK \\ \end{array}}}\,+2{{H}_{2}}O\xrightarrow{\text{Electrolysis}}\underset{\text{Ethene}}{\mathop{\begin{array}{*{35}{l}} C{{H}_{2}} \\ \,| \\ C{{H}_{2}} \\ \end{array}}}\,+2C{{O}_{2}}+{{H}_{2}}+2KOH\]
(vii) From esters [Pyrolysis of ester] :
\[C{{H}_{3}}-CO-\underset{C{{H}_{2}}-C{{H}_{2}}}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|\,\,\,\,\,\,\,}{\mathop{O\,\,\,\,\,\,\,\,\,\,\,\,H\,\,\,\,\,\,}}\,}}\,\underset{liq.\,{{N}_{2}}}{\mathop{\begin{align} & \\ & \xrightarrow{\text{Glass}\,\text{wool}\,{{450}^{o}}} \\ \end{align}}}\,\underset{C{{H}_{2}}=C{{H}_{2}}}{\mathop{\underset{+}{\mathop{C{{H}_{3}}-COOH}}\,}}\,\]
(viii) Pyrolysis of quaternary ammonium compounds :
\[\underset{\underset{\text{hydroxide}}{\mathop{\text{Tetraethyl}\,\text{ammonium}}}\,}{\mathop{{{({{C}_{2}}{{H}_{5}})}_{4}}\overset{+}{\mathop{N}}\,\overset{-}{\mathop{OH}}\,}}\,\xrightarrow{heat}\underset{\underset{\text{(Tert}\text{.}\,\text{amine)}}{\mathop{\text{Triethylamine}}}\,}{\mathop{{{({{C}_{2}}{{H}_{5}})}_{3}}N}}\,+\underset{\text{Ethene}}{\mathop{{{C}_{2}}{{H}_{4}}}}\,+{{H}_{2}}O\]
(ix) Action of copper alkyl on vinyl chloride :
\[\underset{\text{Vinyl}\,\text{chloride}}{\mathop{{{H}_{2}}C=CHCl}}\,\xrightarrow{Cu{{R}_{2}}}{{H}_{2}}C=CHR\]
(x) By Grignard reagents :
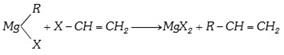 (xi) The wittig reaction :
\[{{(Ph)}_{3}}P=C{{H}_{2}}+\underset{O\,\,\,}{\mathop{\underset{|\,|\,\,\,\,}{\mathop{CH}}\,}}\,-R\xrightarrow{{}}{{(Ph)}_{3}}P=O+R-\underset{C{{H}_{2}}}{\mathop{\underset{|\,|\,\,\,\,\,\,}{\mathop{CH\,}}\,}}\,\]
\[{{(Ph)}_{3}}P=CH-R+\overset{O\,\,\,}{\mathop{\overset{|\,|\,\,\,\,}{\mathop{CH}}\,}}\,-R\xrightarrow{{}}{{(Ph)}_{3}}P=O+R-CH=CH-R\]
(xii) From \[\beta \]bromo ether [Boord synthesis]
(xi) The wittig reaction :
\[{{(Ph)}_{3}}P=C{{H}_{2}}+\underset{O\,\,\,}{\mathop{\underset{|\,|\,\,\,\,}{\mathop{CH}}\,}}\,-R\xrightarrow{{}}{{(Ph)}_{3}}P=O+R-\underset{C{{H}_{2}}}{\mathop{\underset{|\,|\,\,\,\,\,\,}{\mathop{CH\,}}\,}}\,\]
\[{{(Ph)}_{3}}P=CH-R+\overset{O\,\,\,}{\mathop{\overset{|\,|\,\,\,\,}{\mathop{CH}}\,}}\,-R\xrightarrow{{}}{{(Ph)}_{3}}P=O+R-CH=CH-R\]
(xii) From \[\beta \]bromo ether [Boord synthesis]
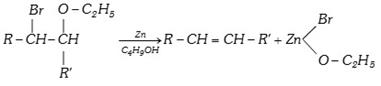 (2) Physical Properties
(i) Alkenes are colourless and odourless.
(ii) These are insoluble in water and soluble in organic solvents.
(iii) Physical state
\[{{C}_{1}}-{{C}_{4}}\xrightarrow{{}}\] gas
\[{{C}_{4}}-{{C}_{16}}\xrightarrow{{}}\] liquid
\[>\,\,\,{{C}_{17}}\xrightarrow{{}}\] solid wax
(iv) B.P. and M.P. decreases with increasing branches in alkene.
(v) The melting points of cis isomers are lower than trans isomers because cis isomer is less symmetrical than trans. Thus trans packs more tightly in the crystal lattice and hence has a higher melting point.
(vi) The boiling points of cis isomers are higher than trans isomers because cis-alkenes has greater polarity (Dipole moment) than trans one.
(vii) These are lighter than water.
(viii) Dipole moment : Alkenes are weakly polar. The, \[\pi -\]electron’s of the double bond. Can be easily polarized. Therefore, their dipole moments are higher than those of alkanes.
(3) Chemical properties
(i) Francis experiment : According to Francis electrophile first attacks on olefinic bond.
(2) Physical Properties
(i) Alkenes are colourless and odourless.
(ii) These are insoluble in water and soluble in organic solvents.
(iii) Physical state
\[{{C}_{1}}-{{C}_{4}}\xrightarrow{{}}\] gas
\[{{C}_{4}}-{{C}_{16}}\xrightarrow{{}}\] liquid
\[>\,\,\,{{C}_{17}}\xrightarrow{{}}\] solid wax
(iv) B.P. and M.P. decreases with increasing branches in alkene.
(v) The melting points of cis isomers are lower than trans isomers because cis isomer is less symmetrical than trans. Thus trans packs more tightly in the crystal lattice and hence has a higher melting point.
(vi) The boiling points of cis isomers are higher than trans isomers because cis-alkenes has greater polarity (Dipole moment) than trans one.
(vii) These are lighter than water.
(viii) Dipole moment : Alkenes are weakly polar. The, \[\pi -\]electron’s of the double bond. Can be easily polarized. Therefore, their dipole moments are higher than those of alkanes.
(3) Chemical properties
(i) Francis experiment : According to Francis electrophile first attacks on olefinic bond.
 (ii) Reaction with hydrogen :
\[R-\overset{H\,\,\,\,H}{\mathop{\overset{|\,\,\,\,\,\,\,\,|}{\mathop{C=C}}\,}}\,-R+{{H}_{2}}\xrightarrow{Ni}R-\underset{H\,\,\,H}{\mathop{\underset{|\,\,\,\,\,\,\,|}{\mathop{\overset{H\,\,\,H}{\mathop{\overset{|\,\,\,\,\,\,\,|}{\mathop{C-C}}\,}}\,}}\,}}\,-R\]
(iii) Reduction of alkene via hydroboration : Alkene can be converted into alkane by protolysis
\[RCH=C{{H}_{2}}\xrightarrow{H-B{{H}_{2}}}{{(R-C{{H}_{2}}-C{{H}_{2}})}_{3}}B\]
\[\xrightarrow{{{H}^{+}}/{{H}_{2}}O}R-C{{H}_{2}}-C{{H}_{3}}\]
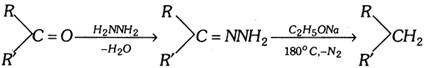
Hydroboration : Alkene give addition reaction with diborane which called hydroboration. In more...
(ii) Reaction with hydrogen :
\[R-\overset{H\,\,\,\,H}{\mathop{\overset{|\,\,\,\,\,\,\,\,|}{\mathop{C=C}}\,}}\,-R+{{H}_{2}}\xrightarrow{Ni}R-\underset{H\,\,\,H}{\mathop{\underset{|\,\,\,\,\,\,\,|}{\mathop{\overset{H\,\,\,H}{\mathop{\overset{|\,\,\,\,\,\,\,|}{\mathop{C-C}}\,}}\,}}\,}}\,-R\]
(iii) Reduction of alkene via hydroboration : Alkene can be converted into alkane by protolysis
\[RCH=C{{H}_{2}}\xrightarrow{H-B{{H}_{2}}}{{(R-C{{H}_{2}}-C{{H}_{2}})}_{3}}B\]
\[\xrightarrow{{{H}^{+}}/{{H}_{2}}O}R-C{{H}_{2}}-C{{H}_{3}}\]
Hydroboration : Alkene give addition reaction with diborane which called hydroboration. In more... 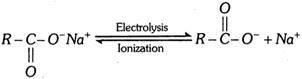 At anode [Oxidation] :
\[\underset{O\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|\,|\,\,\,\,\,\,\,\,\,\,\,}{\mathop{2R-C-{{O}^{-}}-2{{e}^{-}}}}\,}}\,\xrightarrow{{}}2R-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,-\overset{\bullet }{\mathop{O}}\,\xrightarrow{{}}2\overset{\bullet }{\mathop{R}}\,+2C{{O}_{2}}\]
\[2\overset{\bullet }{\mathop{R}}\,\xrightarrow{{}}R-R\] (alkane)
At cathode [Reduction] :
\[2N{{a}^{+}}+2{{e}^{-}}\xrightarrow{{}}2Na\xrightarrow{2{{H}_{2}}O}2NaOH+{{H}_{2}}\] \[(\uparrow )\]
At anode [Oxidation] :
\[\underset{O\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|\,|\,\,\,\,\,\,\,\,\,\,\,}{\mathop{2R-C-{{O}^{-}}-2{{e}^{-}}}}\,}}\,\xrightarrow{{}}2R-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,-\overset{\bullet }{\mathop{O}}\,\xrightarrow{{}}2\overset{\bullet }{\mathop{R}}\,+2C{{O}_{2}}\]
\[2\overset{\bullet }{\mathop{R}}\,\xrightarrow{{}}R-R\] (alkane)
At cathode [Reduction] :
\[2N{{a}^{+}}+2{{e}^{-}}\xrightarrow{{}}2Na\xrightarrow{2{{H}_{2}}O}2NaOH+{{H}_{2}}\] \[(\uparrow )\]
 (xi) Hydroboration of alkenes
(a) On treatment with acetic acid
\[\underset{\text{Alkene}}{\mathop{R-CH=C{{H}_{2}}}}\,\xrightarrow{{{B}_{2}}{{H}_{6}}}\underset{\text{Trialkyl borane}}{\mathop{{{(R-C{{H}_{2}}-C{{H}_{2}})}_{3}}B}}\,\xrightarrow{C{{H}_{3}}COOH}\]\[\underset{\text{Alkane}}{\mathop{R-C{{H}_{2}}-C{{H}_{3}}}}\,\]
(b) Coupling of alkyl boranes by means of silver nitrate
\[6[R-CH=C{{H}_{2}}]\xrightarrow{2{{B}_{2}}{{H}_{6}}}{{[2R-C{{H}_{2}}-C{{H}_{2}}-]}_{3}}B\underset{NaOH}{\mathop{\xrightarrow{AgN{{O}_{3}}\,{{25}^{o}}C}}}\,\]\[3[RC{{H}_{2}}C{{H}_{2}}-C{{H}_{2}}C{{H}_{2}}R]\]
(2) Physical Properties
(i) Physical state : Alkanes are colourless, odourless and tasteless.
Alkanes State
\[{{C}_{1}}-{{C}_{4}}\] Gaseous state
\[{{C}_{5}}-{{C}_{17}}\] Liquid state [Except neo pentane which is gas]
\[{{C}_{18}}\] and above Solid like waxes
(ii) Density : Alkanes are lighter than water.
(iii) Solubility : Insoluble in water, soluble in organic solvents, solubility \[\propto \frac{1}{\text{Molecular mass}}\]
(iv) Boiling points and Melting points : Melting points and boiling points. \[\propto \] Molecular mass \[\propto \frac{1}{\text{No}\text{.}\,\text{of}\,\text{branches}}\]
(xi) Hydroboration of alkenes
(a) On treatment with acetic acid
\[\underset{\text{Alkene}}{\mathop{R-CH=C{{H}_{2}}}}\,\xrightarrow{{{B}_{2}}{{H}_{6}}}\underset{\text{Trialkyl borane}}{\mathop{{{(R-C{{H}_{2}}-C{{H}_{2}})}_{3}}B}}\,\xrightarrow{C{{H}_{3}}COOH}\]\[\underset{\text{Alkane}}{\mathop{R-C{{H}_{2}}-C{{H}_{3}}}}\,\]
(b) Coupling of alkyl boranes by means of silver nitrate
\[6[R-CH=C{{H}_{2}}]\xrightarrow{2{{B}_{2}}{{H}_{6}}}{{[2R-C{{H}_{2}}-C{{H}_{2}}-]}_{3}}B\underset{NaOH}{\mathop{\xrightarrow{AgN{{O}_{3}}\,{{25}^{o}}C}}}\,\]\[3[RC{{H}_{2}}C{{H}_{2}}-C{{H}_{2}}C{{H}_{2}}R]\]
(2) Physical Properties
(i) Physical state : Alkanes are colourless, odourless and tasteless.
Alkanes State
\[{{C}_{1}}-{{C}_{4}}\] Gaseous state
\[{{C}_{5}}-{{C}_{17}}\] Liquid state [Except neo pentane which is gas]
\[{{C}_{18}}\] and above Solid like waxes
(ii) Density : Alkanes are lighter than water.
(iii) Solubility : Insoluble in water, soluble in organic solvents, solubility \[\propto \frac{1}{\text{Molecular mass}}\]
(iv) Boiling points and Melting points : Melting points and boiling points. \[\propto \] Molecular mass \[\propto \frac{1}{\text{No}\text{.}\,\text{of}\,\text{branches}}\]
| more...
(1) Knocking : The metallic sound during working of an internal combustion engine is termed as knocking.
“The greater the compression greater will be efficiency of engine.” The fuel which has minimum knocking property is always preferred.
The tendency to knock falls off in the following order : Straight chain alkanes > branched chain alkanes > olefins > cyclo alkanes > aromatic hydrocarbons.
(2) Octane number : It is used for measuring the knocking character of fuel used in petrol engine. The octane number of a given sample may be defined as the percentage by volume of iso-octane present in a mixture of iso-octane and n-heptane which has the same knocking performance as the fuel itself.
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\]
n-heptane; octane no. = 0
\[\underset{\,\,\,\,\,\,C{{H}_{3}}}{\mathop{\underset{|}{\mathop{\overset{\,\,\,\,\,\,\,\,C{{H}_{3}}}{\mathop{\overset{|}{\mathop{C{{H}_{3}}-C-C{{H}_{2}}}}\,}}\,}}\,}}\,\overset{C{{H}_{3}}\,\,\,}{\mathop{\overset{|\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{-C-C{{H}_{3}}}}\,}}\,\] ; Octane no. = 100
2, 2, 4-Trimethyl pentane or Iso-octane.
For example : a given sample has the knocking performance equivalent to a mixture containing 60% iso-octane and 40% heptane. The octane number of the gasoline is, therefore, 60.
Presence of following types of compounds increases the octane number of gasoline.
(i) In case of straight chain hydrocarbons octane number decreases with increase in the length of the chain.
(ii) Branching of chain increases the value of octane number
(iii) Introduction of double bond or triple bond increases the value of octane number.
(iv) Cyclic alkanes have relatively higher value of octane number.
(v) The octane number of aromatic hydrocarbons are exceptionally high
(vi) By adding gasoline additives (eg TEL)
(3) Antiknock compounds : To reduce the knocking property or to improve the octane number of a fuel certain chemicals are added to it. These are called antiknock compounds. One such compound, which is extensively used, is tetraethyl lead (TEL). TEL is used in the form of following mixture,
TEL = 63%, Ethylene bromide = 26%, Ethylene chloride = 9% and a dye = 2%.
However, there is a disadvantage that the lead is deposited in the engine. To remove the free lead, the ethylene halides are added which combine with lead to form volatile lead halides.
\[\underset{\text{Ethylene bromide}}{\mathop{Pb+Br-C{{H}_{2}}-C{{H}_{2}}-Br}}\,\to \underset{\text{Volatile}}{\mathop{PbB{{r}_{2}}}}\,+\underset{\text{Ethylene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,\]
However, use of TEL in petrol is facing a serious problem of Lead pollution, to avoid this a new compound cyclopenta dienyl manganese carbonyl (called as AK-33-X) is used in developed countries as antiknocking compound.
(4) Other methods of improving octane number of hydrocarbon.
(i) Isomerisation [Reforming] : By passing an alkane over \[AlC{{l}_{3}}\] at \[{{200}^{o}}C\].
\[\underset{\text{(Octane number }=\text{ 62)}}{\mathop{\underset{\text{Pentane}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}}}\,}}\,\underset{{{200}^{o}}C}{\mathop{\xrightarrow{AlC{{l}_{3}}}}}\,\underset{(\text{Octane number}\,=\,\text{90})}{\mathop{\underset{\text{Isopentane}}{\mathop{C{{H}_{3}}\overset{C{{H}_{3}}\,}{\mathop{\overset{|\,\,\,\,\,\,\,\,\,}{\mathop{CHC}}\,}}\,{{H}_{2}}C{{H}_{3}}}}\,}}\,\]
(ii) Alkylation :
\[\underset{\text{Isobutane}}{\mathop{\underset{C{{H}_{3}}\,\,\,}{\overset{C{{H}_{3}}\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,}{\overset{|\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{3}}CH+C{{H}_{2}}}}}\,}}}\,}}\,\underset{\text{Isobutylene}}{\mathop{=\overset{C{{H}_{3}}\,\,\,\,}{\mathop{\overset{|}{\mathop{C}}\,C{{H}_{3}}}}\,}}\,\xrightarrow{{{H}_{2}}S{{O}_{4}}}\underset{\begin{smallmatrix} \text{Iso-octane} \\ \text{(Octane number }=\text{ 100)}\end{smallmatrix}}{\mathop{\underset{\,\,\,\,\,C{{H}_{3}}}{\overset{\,\,\,\,\,C{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{C{{H}_{3}}CC{{H}_{2}}}}}\,}}}\,\overset{C{{H}_{3}}\,\,\,\,\,\,\,}{\mathop{\overset{|\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{CHC{{H}_{3}}}}\,}}\,}}\,\]
(iii) Aromatisation :
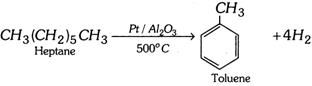 The octane no. of petrol can thus be improved.
The octane no. of petrol can thus be improved.
Organic compounds composed of only carbon and hydrogen are called hydrocarbons. Hydrocarbons are two types
(1) Aliphatic Hydrocarbon (Alkanes, Alkenes and Alkynes).
(2) Aromatic Hydrocarbon (Arenes)
(1) Sources of aliphatic hydrocarbon
Mineral oil or crude oil, petroleum [Petra ® rock; oleum ® oil] is the dark colour oily liquid with offensive odour found at various depths in many regions below the surface of the earth. It is generally found under the rocks of earth’s crust and often floats over salted water.
(2) Composition
(i) Alkanes : found 30 to 70% contain upto 40 carbon atom. Alkanes are mostly straight chain but some are branched chain isomers.
(ii) Cycloalkanes : Found 16 to 64% cycloalkanes present in petroleum are; cyclohexane, methyl cyclopentane etc. cycloalkanes rich oil is called asphaltic oil.
(iii) Aromatic hydrocarbon : found 8 to 15% compound present in petroleum are; Benzene, Toluene, Xylene, Naphthalene etc.
(iv) Sulphur, nitrogen and oxygen compound : Sulphur compound present to the extent of 6% include mercaptans [R-SH] and sulphides [R-S-R]. The unpleasant smell of petroleum is due to sulphur compounds. Nitrogenous compounds are pyridines, quinolines and pyrroles. Oxygen compounds present in petroleum are. Alcohols, Phenols and resins. Compounds like chlorophyll, haemin are also present in it.
(v) Natural gas : It is a mixture of Methane (80%), Ethane (13%), Propane (3%), Butane (1%), Vapours of low boiling pentanes and hexanes (0.5%) and Nitrogen (1.3%). L.P.G. Contain butanes and pentanes and used as cooking gas. It is highly inflammable. This contain, methane, nitrogen and ethane.
(vi) C.N.G. : When natural gas compressed at very high pressure is called compressed natural gas (CNG). Natural gas has octane rating of 130 it consists, mainly of methane and may contain, small amount of ethane and propane.
(3) Theories of origin of petroleum : Theories must explain the following characteristics associated with petroleum,
Its association with brine (sodium chloride solution). The presence of nitrogen and sulphur compounds in it. The presence of chlorophyll and haemin in it. Its optically active nature. Three important theories are as follows.
(i) Mendeleeff’s carbide theory or inorganic theory
(ii) Engler’s theory or organic theory
(iii) Modern theory
(4) Mining of petroleum : Petroleum deposits occurs at varying depth at different places ranging from 500 to 15000 feet. This is brought to the surface by artificial drilling.
(5) Petroleum refining : Separation of useful fractions by fractional distillation is called petroleum refining.
|