 Uses : Starch and its derivatives are used
(i) As the most valuable constituent of food as rice, bread, potato and corn-flour, etc.
(ii) In the manufacture of glucose, dextrin and adhesives (starch paste).
(iii) In paper and textile industry.
(iv) In calico printing as a thickening agent for colours.
(v) Nitro starch is used as an explosive.
(vi) Starch-acetate is a transparent gelatin like mass and is used mainly for making sweets.
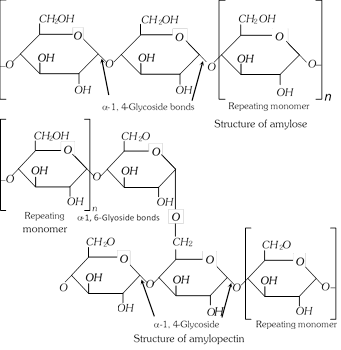
(2) Cellulose : It is found in all plants and so is the most abundant of all carbohydrates. It is the material used to form cell walls and other structural features of the plants. Wood is about 50% cellulose and the rest is lignin. Cotton and paper are largely composed of cellulose.
Pure cellulose is obtained by successively treating cotton, wool, flax or paper with dilute alkali, dilute \[HCl\] or \[HF\]. This treatment removes mineral matter, water, alcohol and ether. Cellulose is left behind as a white amorphous powder.
Cellulose is insoluble in water and in most of the organic solvents. It decomposes on heating but does not melt. It dissolves in ammonical copper hydroxide solution (Schwitzer’s reagent). Cellulose also dissolves in a solution of zinc chloride in hydrochloric acid.
When it is treated with concentrated \[{{H}_{2}}S{{O}_{4}}\] in cold, it slowly passes into solution. The solution when diluted with water, a starch like substance amyloid is precipitated and is called parchment paper. When more...
Uses : Starch and its derivatives are used
(i) As the most valuable constituent of food as rice, bread, potato and corn-flour, etc.
(ii) In the manufacture of glucose, dextrin and adhesives (starch paste).
(iii) In paper and textile industry.
(iv) In calico printing as a thickening agent for colours.
(v) Nitro starch is used as an explosive.
(vi) Starch-acetate is a transparent gelatin like mass and is used mainly for making sweets.
(2) Cellulose : It is found in all plants and so is the most abundant of all carbohydrates. It is the material used to form cell walls and other structural features of the plants. Wood is about 50% cellulose and the rest is lignin. Cotton and paper are largely composed of cellulose.
Pure cellulose is obtained by successively treating cotton, wool, flax or paper with dilute alkali, dilute \[HCl\] or \[HF\]. This treatment removes mineral matter, water, alcohol and ether. Cellulose is left behind as a white amorphous powder.
Cellulose is insoluble in water and in most of the organic solvents. It decomposes on heating but does not melt. It dissolves in ammonical copper hydroxide solution (Schwitzer’s reagent). Cellulose also dissolves in a solution of zinc chloride in hydrochloric acid.
When it is treated with concentrated \[{{H}_{2}}S{{O}_{4}}\] in cold, it slowly passes into solution. The solution when diluted with water, a starch like substance amyloid is precipitated and is called parchment paper. When more... 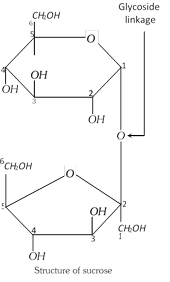 (ii) Uses
(a) As a sweetening agent for various food preparations, jams, syrups sweets, etc.
(b) In the manufacture of sucrose octa-acetate required to denature alcohol, to make paper transparent and to make anhydrous adhesives.
(2) Inversion of cane-sugar : The hydrolysis of sucrose by boiling with a mineral acid or by enzyme invertase, produces a mixture of equal molecules of D-glucose and D-fructose.
\[\underset{\text{Sucrose}}{\mathop{{{C}_{12}}{{H}_{22}}{{O}_{11}}}}\,+{{H}_{2}}O\xrightarrow{{{H}^{+}}}\underset{\text{(This mixture is laevorotatory)}}{\mathop{\underset{\text{D-Glucose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,+\underset{\text{D-Fructose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,}}\,\]
Sucrose solution is dextrorotatory. Its specific rotation is \[+{{66.5}^{o}}.\] But on hydrolysis, it becomes laevorotatory. The specific rotation of D-glucose is \[+{{52}^{o}}\] and of D-fructose is \[-{{92}^{o}}.\] Therefore, the net specific rotation of an equimolar mixture of D-glucose and D-fructose is.
\[\frac{+{{52}^{o}}-{{92}^{o}}}{2}=-{{20}^{o}}\]
Thus, in the process of hydrolysis of sucrose, the specific rotation changes from \[+\,\,66.5{}^\circ \] to \[-\,\,20{}^\circ \], i.e., from dextro it becomes laevo and it is said that inversion has taken place. The process of hydrolysis of sucrose is thus termed as inversion of sugar and the hydrolysed mixture having equal molar quantities of D-glucose and D-fructose is called invert sugar. The enzyme that brings the inversion is named as invertase.
Distinction between glucose and sucrose
(ii) Uses
(a) As a sweetening agent for various food preparations, jams, syrups sweets, etc.
(b) In the manufacture of sucrose octa-acetate required to denature alcohol, to make paper transparent and to make anhydrous adhesives.
(2) Inversion of cane-sugar : The hydrolysis of sucrose by boiling with a mineral acid or by enzyme invertase, produces a mixture of equal molecules of D-glucose and D-fructose.
\[\underset{\text{Sucrose}}{\mathop{{{C}_{12}}{{H}_{22}}{{O}_{11}}}}\,+{{H}_{2}}O\xrightarrow{{{H}^{+}}}\underset{\text{(This mixture is laevorotatory)}}{\mathop{\underset{\text{D-Glucose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,+\underset{\text{D-Fructose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,}}\,\]
Sucrose solution is dextrorotatory. Its specific rotation is \[+{{66.5}^{o}}.\] But on hydrolysis, it becomes laevorotatory. The specific rotation of D-glucose is \[+{{52}^{o}}\] and of D-fructose is \[-{{92}^{o}}.\] Therefore, the net specific rotation of an equimolar mixture of D-glucose and D-fructose is.
\[\frac{+{{52}^{o}}-{{92}^{o}}}{2}=-{{20}^{o}}\]
Thus, in the process of hydrolysis of sucrose, the specific rotation changes from \[+\,\,66.5{}^\circ \] to \[-\,\,20{}^\circ \], i.e., from dextro it becomes laevo and it is said that inversion has taken place. The process of hydrolysis of sucrose is thus termed as inversion of sugar and the hydrolysed mixture having equal molar quantities of D-glucose and D-fructose is called invert sugar. The enzyme that brings the inversion is named as invertase.
Distinction between glucose and sucrose
| Rubber | Monomers | Formula | Applications |
| (i) Neoprene rubber | \[\underset{\text{Chloroprene}}{\mathop{C{{H}_{2}}=\underset{Cl\,}{\mathop{\underset{|}{\mathop{C}}\,-}}\,CH=C{{H}_{2}}}}\,\] | \[{{\left( -C{{H}_{2}}-\underset{Cl\,}{\mathop{\underset{|}{\mathop{C}}\,=}}\,CH-C{{H}_{2}}- \right)}_{n}}\] | Making automobile, refrigerator parts and electric wire. |
| (ii) Styrene Butadiene Rubber (SBR) or Buna-S | 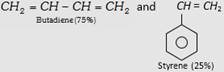 |
 |
Making of tyre and other mechanical rubber goods. |
| (iii) Butyl rubber | 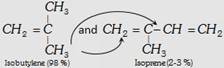 |
\[{{\left( -C{{H}_{2}}-\overset{C{{H}_{3}}\,\,\,\,\,\,}{\mathop{\overset{|}{\mathop{C}}\,=CH}}\,-C{{H}_{2}}-\underset{C{{H}_{3}}\,\,\,\,\,\,\,}{\overset{C{{H}_{3}}\,\,\,\,\,\,\,}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-C{{H}_{2}}}}}\,- \right)}_{n}}\] | Making of toys, tyre, tube etc. |
| (iv) Nitrile rubber or Buna N or GRA | \[\underset{\text{Butadiene }(75%)}{\mathop{C{{H}_{2}}=CH-CH=C{{H}_{2}}}}\,\]and \[\underset{\text{Acrylonitrile }(25%)}{\mathop{C{{H}_{2}}=CH-CN}}\,\] | \[{{\left( -C{{H}_{2}}-\underset{CN}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-C{{H}_{2}}-CH=CH-C{{H}_{2}}- \right)}_{n}}\] | more...
It is a polymer which is capable of returning to its original length, shape or size after being stretched or deformed. It is the example of elastomer. Rubber are of two types.
(1) Natural rubber
(2) Synthetic rubber
(1) Natural rubber : It is obtained as latex from rubber trees. The latex is coagulated with acetic acid or formic acid. The coagulated mass is then squeezed.
The raw natural rubber is a soft gummy and sticky mass. It is insoluble in water, dil. Acids and alkalies but soluble in benzene, chloroform, ether, petrol and carbon disulphide. It absorb a large amount of water. It has low elasticity and tensile strength.
Destructive distillation of natural rubber gives mainly isoprene (2-methyl butadiene).
Thus isoprene is a monomer of natural rubber the no. of isoprene unit are 11,000 to 20,000 which linked together in a chain.
\[\underset{\text{Isopreme}}{\mathop{nC{{H}_{2}}=\overset{C{{H}_{3}}\,\,\,\,\,}{\mathop{\overset{|}{\mathop{C}}\,-CH}}\,}}\,=C{{H}_{2}}\xrightarrow{\text{Polymerisation}}\]\[\underset{\text{Natural rubber}}{\mathop{{{\left[ -C{{H}_{2}}-\overset{C{{H}_{3}}\,\,\,\,\,\,\,}{\mathop{\overset{|}{\mathop{C}}\,=CH}}\,-C{{H}_{2}}- \right]}_{n}}}}\,\]
(2) Synthetic rubber : The synthetic rubber is obtained by polymerising certain organic compounds which may have properties similar to rubber and some desirable properties. Most of these are derived from butadiene derivatives and contain carbon-carbon double bonds. The synthetic rubbers are either homopolymers of 1, 3 butadiene or copolymer in which one of the monomers is 1, 3 butadiene or its derivative so that the polymer has the availability of double bonds for its vulcanization. Some important examples are Neoprene, styrene, butadiene rubber (SBR) thiokol, silicones, polyurethane, rubber etc.
Vulcanization of rubber : The process of heating natural rubber with sulphur to improve its properties is called vulcanization. Vulcanization was introduced by Charles Goodyear.
Although natural rubber is thermoplastic substance in which there are no cross link between the polymer chain and it on vulcanization set into a given shape which is retained.
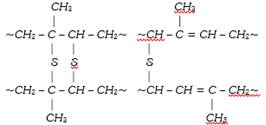 The vulcanization process performed originally was slow. Now a days, some additives such as zinc oxide etc. are used to accelerate the rate of vulcanization. During vulcanization, sulphur cross links are formed (figure) the double bonds in the rubber molecule acts as reactive sites. The allylic\[-C{{H}_{2}}\], alpha to double bond is also very reactive. During vulcanization, sulphur forms cross links at these reactive sites. As a result, rubber gets stiffened and intermolecular movement of rubber springs is prevented resulting in physical character of rubber. The extent of stiffness of vulcanized rubber depend upon the amount of sulphur added. For example about 5% sulphur is used for making tyre rubber while 30% of the sulphur is used for making battery case rubber.
In a polymer, the chains are normally tangled up with each other. When the rubber is stretched, the chains straighten out to some extent. The chains cannot slip past each other because of the polysulphide bridges. Thus, rubber can be stretched only to a limited extent. When the tension is removed, the chains try to coil up more...
The vulcanization process performed originally was slow. Now a days, some additives such as zinc oxide etc. are used to accelerate the rate of vulcanization. During vulcanization, sulphur cross links are formed (figure) the double bonds in the rubber molecule acts as reactive sites. The allylic\[-C{{H}_{2}}\], alpha to double bond is also very reactive. During vulcanization, sulphur forms cross links at these reactive sites. As a result, rubber gets stiffened and intermolecular movement of rubber springs is prevented resulting in physical character of rubber. The extent of stiffness of vulcanized rubber depend upon the amount of sulphur added. For example about 5% sulphur is used for making tyre rubber while 30% of the sulphur is used for making battery case rubber.
In a polymer, the chains are normally tangled up with each other. When the rubber is stretched, the chains straighten out to some extent. The chains cannot slip past each other because of the polysulphide bridges. Thus, rubber can be stretched only to a limited extent. When the tension is removed, the chains try to coil up more...
(1) Chain growth or addition polymerisation : It involve a series of reaction each of which consumes a reactive particle and produces another similar one. The reactive particle may be free radicals or ion (cation or anion) to which monomers get added by a chain reaction. The polymers thus formed are known as chain growth polymers. Chain growth polymerisation is an important reaction of alkenes and conjugated dienes or indeed of all kinds of compounds that contain carbon-carbon double bond polythene, polypropylene, polybutadiene, teflon PVC, polystyrene are some of chain growth polymers. It is based on three mechanism
(i) Free radical mechanism
(ii) Cation mechanism
(iii) Anion mechanism
Each mechanism of polymerisation reaction involves an initiator of their corresponding nature. The addition polymerisation reaction is very rapid and is also characterized by three steps i.e. chain initiation, chain propogation and chain termination step.
(i) Free-radical mechanism : Free-radical polymerisation is initiated by organic peroxide or other reagents which decompose to give free radicals. Following steps are involved.
(a) Chain initiation : Organic peroxides undergo homolytic fission to form free radicals.
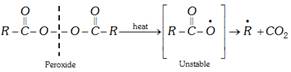 (b) Chain propagation : Free radical adds to an alkene molecule to form a new free radical.
(b) Chain propagation : Free radical adds to an alkene molecule to form a new free radical.
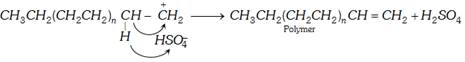 (iii) Anionic polymerisation : This type of polymerisation is initiated by anion (Bases nucleophiles) it proceeds through the formation of carbanion. The initiation may be brought about by \[{{K}^{+}}\bar{N}{{H}_{2}}\] of \[{{L}^{+}}N{{\bar{H}}_{2}}\].
The following steps are involved
(a) Chain initiation :
(iii) Anionic polymerisation : This type of polymerisation is initiated by anion (Bases nucleophiles) it proceeds through the formation of carbanion. The initiation may be brought about by \[{{K}^{+}}\bar{N}{{H}_{2}}\] of \[{{L}^{+}}N{{\bar{H}}_{2}}\].
The following steps are involved
(a) Chain initiation :
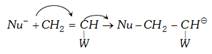 (b) more...
(b) more...
(1) Classification based on source of availability : They are classified as
(i) Natural polymers (ii) Synthetic polymers (iii) Semi-synthetic polymers
(i) Natural polymers : The polymers obtained from nature (plants and animals) are called natural polymers. These polymers are very essential for life. They are as under.
(a) Starch : It is polymer of glucose and it is food reserve of plant.
(b) Cellulose : It is also a polymer of glucose. It is a chief structural material of the plant both starch and cellulose are made by plants from glucose produced during photosynthesis.
(c) Proteins : These are polymers of a-amino acids, they have generally 20 to 1000 \[\alpha \] amino acid joined together in a highly organized arrangement. These are building blocks of animal body and constitute an essential part of our food.
(d) Nucleic acids : These are polymers of various nucleotides. For example RNA and DNA are common nucleotides.
The diazonium salts have the general formula \[ArN_{2}^{+}{{X}^{}}\], where X– may be an anion like Cl–, Br– etc. and the group \[N_{2}^{+}(-N\equiv {{N}^{+}})\] is called diazonium ion group.
(1) Nomenclature : The diazonium salts are named by adding the word diazonium to the name of the parent aromatic compound to which they are related followed by the name of the anion. For example,
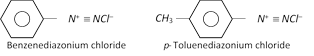
 The diazonium salt may contain other anions also such as \[NO_{3}^{},HSO_{4}^{},B{{F}_{4}}\] etc.
The diazonium salt may contain other anions also such as \[NO_{3}^{},HSO_{4}^{},B{{F}_{4}}\] etc.
 (2) Preparation of diazonium salts :
\[NaN{{O}_{2}}+HCl\to NaCl+HONO\]
(2) Preparation of diazonium salts :
\[NaN{{O}_{2}}+HCl\to NaCl+HONO\]
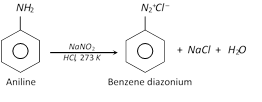 The reaction of converting aromatic primary amine to diazonium salt is called diazotisation.
(3) Physical properties of diazonium salts
(i) Diazonium salts are generally colourless, crystalline solids.
(ii) These are readily soluble in water but less soluble in alcohol.
(iii) They are unstable and explode in dry state. Therefore, they are generally used in solution state.
(iv) Their aqueous solutions are neutral to litmus and conduct electricity due to the presence of ions.
(4) Chemical properties of diazonium salts
(i) Substitution reaction : In substitution or replacement reactions, nitrogen of diazonium salts is lost as \[{{N}_{2}}\] and different groups are introduced in its place.
(a) Replacement by \[-OH\] group
The reaction of converting aromatic primary amine to diazonium salt is called diazotisation.
(3) Physical properties of diazonium salts
(i) Diazonium salts are generally colourless, crystalline solids.
(ii) These are readily soluble in water but less soluble in alcohol.
(iii) They are unstable and explode in dry state. Therefore, they are generally used in solution state.
(iv) Their aqueous solutions are neutral to litmus and conduct electricity due to the presence of ions.
(4) Chemical properties of diazonium salts
(i) Substitution reaction : In substitution or replacement reactions, nitrogen of diazonium salts is lost as \[{{N}_{2}}\] and different groups are introduced in its place.
(a) Replacement by \[-OH\] group
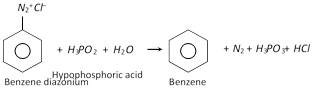
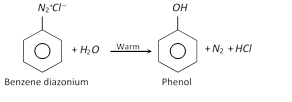 (b) Replacement by hydrogen
(b) Replacement by hydrogen
 (c) Replacement by \[-Cl\] group
(c) Replacement by \[-Cl\] group
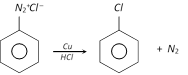
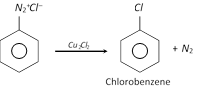 This reaction is called Sandmeyer reaction.
When the diazonium salt solution is warmed with copper powder and the corresponding halogen acid, the respective halogen is introduced. The reaction is a modified form of Sandmeyer reaction and is known as Gattermann reaction.
This reaction is called Sandmeyer reaction.
When the diazonium salt solution is warmed with copper powder and the corresponding halogen acid, the respective halogen is introduced. The reaction is a modified form of Sandmeyer reaction and is known as Gattermann reaction.
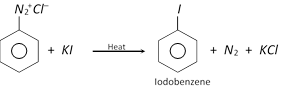
 (d) Replacement by iodo \[(-I)\] group
(d) Replacement by iodo \[(-I)\] group
 (e) Replacement by \[-F\] group
(e) Replacement by \[-F\] group
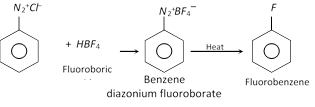 This reaction is called Balz Schiemann reaction.
(f) Replacement by Cyano \[(-CN)\]group
This reaction is called Balz Schiemann reaction.
(f) Replacement by Cyano \[(-CN)\]group
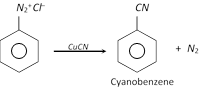 The nitriles can be hydrolysed to acids.
The nitriles can be hydrolysed to acids.
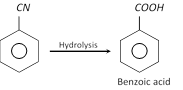 This method of preparing carboxylic acids is more useful than carbonation of Grignard reagents.
(g) Replacement by \[-N{{O}_{2}}\] group
This method of preparing carboxylic acids is more useful than carbonation of Grignard reagents.
(g) Replacement by \[-N{{O}_{2}}\] group
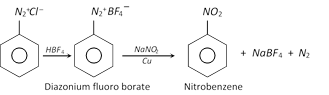 (h) Replacement by thio \[(-SH)\] group
(h) Replacement by thio \[(-SH)\] group
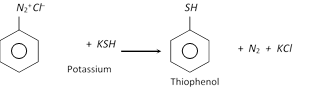 (ii) Coupling reactions : The diazonium ion acts as an electrophile because there is positive charge on terminal nitrogen. It can react with nucleophilic aromatic compounds \[(Ar-H)\] activated by electron donating groups (\[-OH\] and \[-N{{H}_{2}}\]), which as strong nucleophiles react with aromatic diazonium salts. Therefore, benzene diazonium chloride couples with electron rich aromatic more...
(ii) Coupling reactions : The diazonium ion acts as an electrophile because there is positive charge on terminal nitrogen. It can react with nucleophilic aromatic compounds \[(Ar-H)\] activated by electron donating groups (\[-OH\] and \[-N{{H}_{2}}\]), which as strong nucleophiles react with aromatic diazonium salts. Therefore, benzene diazonium chloride couples with electron rich aromatic more... Current Affairs CategoriesArchive
Trending Current Affairs
You need to login to perform this action. |