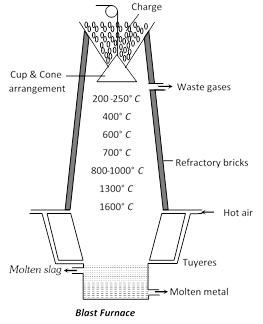
 (2) Reverberatory Furnace : In this furnace fuel burns in a separate part and does not mix with the charge. The furnace may be divided into 3 parts,
(i) Fire Grate : It is on one side where the fuel burns.
(ii) Flue or Chimney : It is on the other side of the fire grate. The waste gases escape through it.
(iii) Hearth : It is the middle part of the furnace where the charge is heated with the flames and hot gases.
The material to be heated is placed on the hearth or bed of the furnace and is heated by the hot gases or flames produced by the burning of fuel. The waste gases escape out of the chimney. Since the fuel does not come in contact with the charge, the furnace is very suitable for calcination and roasting and is employed for both oxidising and reducing purposes. For oxidation, the material is heated by the current of hot air while for reduction the material is mixed with coke and heated. The furnace find wide application in the extractive metallurgy.
(2) Reverberatory Furnace : In this furnace fuel burns in a separate part and does not mix with the charge. The furnace may be divided into 3 parts,
(i) Fire Grate : It is on one side where the fuel burns.
(ii) Flue or Chimney : It is on the other side of the fire grate. The waste gases escape through it.
(iii) Hearth : It is the middle part of the furnace where the charge is heated with the flames and hot gases.
The material to be heated is placed on the hearth or bed of the furnace and is heated by the hot gases or flames produced by the burning of fuel. The waste gases escape out of the chimney. Since the fuel does not come in contact with the charge, the furnace is very suitable for calcination and roasting and is employed for both oxidising and reducing purposes. For oxidation, the material is heated by the current of hot air while for reduction the material is mixed with coke and heated. The furnace find wide application in the extractive metallurgy.
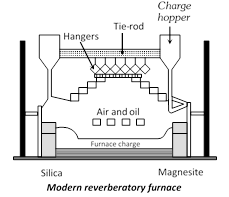 (3) Electric Furnace : The fuel burnt furnaces described in this chapter produce temperature in the range of \[1000-{{1500}^{o}}C\]. Although these furnaces have the great utility in the extraction of metals yet these are unsuitable where higher temperatures are needed. One commonly used electric furnace is Heroult’s furnace shown in fig. It consists of a steel shell lined inside with dolomite or magnesite. It is provided with movable water jacketed electrodes suspended from the roof or from the sides. Heat is generated by striking an arc between the electrodes, thereby, a temperature of over \[{{3000}^{o}}C\] may be reached. The charge melts and the impurities e.g., Si, Mn, P and S etc. present in the more...
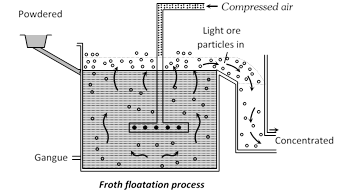
(3) Electric Furnace : The fuel burnt furnaces described in this chapter produce temperature in the range of \[1000-{{1500}^{o}}C\]. Although these furnaces have the great utility in the extraction of metals yet these are unsuitable where higher temperatures are needed. One commonly used electric furnace is Heroult’s furnace shown in fig. It consists of a steel shell lined inside with dolomite or magnesite. It is provided with movable water jacketed electrodes suspended from the roof or from the sides. Heat is generated by striking an arc between the electrodes, thereby, a temperature of over \[{{3000}^{o}}C\] may be reached. The charge melts and the impurities e.g., Si, Mn, P and S etc. present in the more...  (ii) Froth floatation process : In some cases for example, sulphides ores of copper, zinc and lead concentration is brought by this method. In this method advantage is taken of the preferential wetting of the ore by an oil. The finely ground ore is taken in a tank containing water and 1% of pine oil or terpentine oil. A strong current of air is blown through the suspension, producing a heavy froth or foam on the surface. The metal sulphide is wetted by the oil but the gangues is not and the sulphide-oil mixture is carried to the surface by films of oil The froth is skimmed off, the gangue settles down on the bottom or remains underneath the froth. By this floatation method it is possible to concentrate over 90% of a sulphite ore to 1/10 of its original bulk.
(ii) Froth floatation process : In some cases for example, sulphides ores of copper, zinc and lead concentration is brought by this method. In this method advantage is taken of the preferential wetting of the ore by an oil. The finely ground ore is taken in a tank containing water and 1% of pine oil or terpentine oil. A strong current of air is blown through the suspension, producing a heavy froth or foam on the surface. The metal sulphide is wetted by the oil but the gangues is not and the sulphide-oil mixture is carried to the surface by films of oil The froth is skimmed off, the gangue settles down on the bottom or remains underneath the froth. By this floatation method it is possible to concentrate over 90% of a sulphite ore to 1/10 of its original bulk.
 (ii) Activators and Depressants more...
(ii) Activators and Depressants more... | Metal | Name of the ore | Composition |
| Pb | Galena | PbS |
| Zn | Zinc blende | ZnS |
| Hg | Cinnabar | HgS |
| Ag | Argentite or silver glance Pyrargyrite or ruby silver | \[A{{g}_{2}}S3A{{g}_{2}}S.S{{b}_{2}}{{S}_{3}}\] |
| Fe | Iron pyrites | \[Fe{{S}_{2}}\] |
| Ni | Kupfer nickel | NiAs |
| Cu | Copper pyrites Chalcocite or Copper glance | \[CuFe{{S}_{2}}C{{u}_{2}}S\] |
You need to login to perform this action.
You will be redirected in
3 sec
