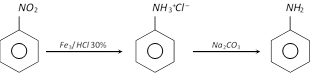
 Aniline is also obtained on a large scale by the action of amine on chlorobenzene at \[{{200}^{o}}C\] under 300-400 atm pressure in presence of cuprous catalyst.
\[2{{C}_{6}}{{H}_{5}}Cl+2N{{H}_{3}}+C{{u}_{2}}O\underset{300-400\,\,atm}{\mathop{\xrightarrow{\ 200{}^\circ C\ }}}\,2{{C}_{6}}{{H}_{5}}N{{H}_{2}}+C{{u}_{2}}C{{l}_{2}}+{{H}_{2}}O\]
Properties Aniline when freshly prepared is a colourless oily liquid (b.p. \[{{184}^{o}}C\]). It has a characteristic unpleasant odour and is not poisonous in nature. It is heavier than water and is only slightly soluble. It is soluble in alcohol, ether and benzene. Its colour changes to dark brown on standing.
It shows all the characteristic reactions discussed earlier.
Uses : (1) It is used in the preparation of diazonium compounds which are used in dye industry.
(2) Anils (Schiff's bases from aniline) are used as antioxidants in rubber industry.
(3) It is used for the manufacture of its some derivatives such as acetamide, sulphanilic acid and sulpha drugs, etc.
(4) It is used as an accelerator in vulcanizing rubber.
Aniline is also obtained on a large scale by the action of amine on chlorobenzene at \[{{200}^{o}}C\] under 300-400 atm pressure in presence of cuprous catalyst.
\[2{{C}_{6}}{{H}_{5}}Cl+2N{{H}_{3}}+C{{u}_{2}}O\underset{300-400\,\,atm}{\mathop{\xrightarrow{\ 200{}^\circ C\ }}}\,2{{C}_{6}}{{H}_{5}}N{{H}_{2}}+C{{u}_{2}}C{{l}_{2}}+{{H}_{2}}O\]
Properties Aniline when freshly prepared is a colourless oily liquid (b.p. \[{{184}^{o}}C\]). It has a characteristic unpleasant odour and is not poisonous in nature. It is heavier than water and is only slightly soluble. It is soluble in alcohol, ether and benzene. Its colour changes to dark brown on standing.
It shows all the characteristic reactions discussed earlier.
Uses : (1) It is used in the preparation of diazonium compounds which are used in dye industry.
(2) Anils (Schiff's bases from aniline) are used as antioxidants in rubber industry.
(3) It is used for the manufacture of its some derivatives such as acetamide, sulphanilic acid and sulpha drugs, etc.
(4) It is used as an accelerator in vulcanizing rubber. 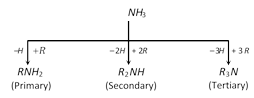 Amines are classified as primary, secondary or tertiary depending on the number of alkyl groups attached to nitrogen atom.
The characteristic groups in primary, secondary and tertiary amines are: \[\underset{\text{(amino)}}{\mathop{N{{H}_{2}}}}\,\]; \[\underset{\text{(imino)}}{\overset{|}{\mathop{NH}}}\,\]; \[\underset{(tert-\text{nitrogen)}}{\mathop{\underset{|}{\overset{|}{\mathop{-N\,\,\,}}}\,}}\,\]
In addition to above amines, tetra-alkyl derivatives similar to ammonium salts also exist which are called quaternary ammonium compounds.
\[N{{H}_{4}}I\]; \[\underset{\begin{smallmatrix} \,\,\,\,\,\,\text{Quaternary} \\ \text{ammonium }\,\text{iodide} \end{smallmatrix}}{\mathop{{{R}_{4}}NI}}\,\]; \[\underset{\begin{smallmatrix} \,\,\,\,\text{Tetramethyl} \\ \text{ammonium iodide} \end{smallmatrix}}{\mathop{{{(C{{H}_{3}})}_{4}}NI}}\,\] or \[\underset{\begin{smallmatrix} \,\,\,\,\text{Tetra-alkyl} \\ \text{ammonium salt} \end{smallmatrix}}{\mathop{{{\left[ R-\underset{\underset{R}{\mathop{|}}\,}{\overset{\overset{R}{\mathop{|}}\,}{\mathop{N}}}\,-R \right]}^{+}}}}\,{{X}^{-}}\]
(1) Simple and mixed amines : Secondary and tertiary amines may be classified as simple or mixed amines according as all the alkyl or aryl groups attached to the nitrogen atom are same or different. For example,
Simple amines : \[\underset{\text{Dimethylamine}}{\mathop{{{(C{{H}_{3}})}_{2}}NH}}\,\]; \[\underset{\text{Triethylamine}}{\mathop{{{(C{{H}_{3}}C{{H}_{2}})}_{3}}N}}\,\]
Mixed amines : \[\underset{\text{Ethylmethylamine}}{\mathop{{{C}_{2}}{{H}_{5}}-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{N}}\,H}}\,}}\,\]; \[\underset{\text{Methylaniline}}{\mathop{{{C}_{6}}{{H}_{5}}-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{N}}\,H}}\,}}\,\]
The aliphatic amines have pyramidal shape with one electron pair. In amines, N undergoes hybridisation.
(2) General methods of preparation
(i) Methods yielding mixture of amines (Primary, secondary and tertiary)
(a) Hofmann's method :The mixture of amines (\[{{1}^{o}},\,\,{{2}^{o}}\] and \[{{3}^{o}}\]) is formed by the alkylation of ammonia with alkyl halides.
\[\underset{\text{Methyliodi}\text{de}}{\mathop{C{{H}_{3}}I}}\,+N{{H}_{3}}\to \underset{\begin{smallmatrix} \text{Methylamine} \\ \,\,\,\,\,\text{(1}{}^\circ \text{)} \end{smallmatrix}}{\mathop{C{{H}_{3}}N{{H}_{2}}}}\,\xrightarrow{C{{H}_{3}}I}(\underset{\begin{smallmatrix} \text{Dimethylamine} \\ \,\,\,\,\,\,\,\text{(2}{}^\circ \text{)} \end{smallmatrix}}{\mathop{C{{H}_{3}}{{)}_{2}}NH}}\,\]
\[\xrightarrow{C{{H}_{3}}I}\underset{\begin{smallmatrix} \text{Trimethylamine} \\ \,\,\,\,\,\,\,\,\,\,\,\text{(3}{}^\circ \text{)} \end{smallmatrix}}{\mathop{{{(C{{H}_{3}})}_{3}}N}}\,\xrightarrow{C{{H}_{3}}I}\underset{\begin{smallmatrix} \,\,\,\,\text{Tetramethyl} \\ \text{ammonium iodide} \end{smallmatrix}}{\mathop{{{(C{{H}_{3}})}_{4}}NI}}\,\]
The primary amine may be obtained in a good yield by using a large excess of ammonia. The process is also termed as ammonolysis of alkyl halides. It is a nucleophilic substitution reaction.
(b) Ammonolysis of alcohols :
\[C{{H}_{3}}OH+N{{H}_{3}}\underset{350{}^\circ C}{\mathop{\xrightarrow{A{{l}_{2}}{{O}_{3}}}}}\,C{{H}_{3}}N{{H}_{2}}\]\[\xrightarrow{C{{H}_{3}}OH}{{(C{{H}_{3}})}_{2}}NH\xrightarrow{C{{H}_{3}}OH}{{(C{{H}_{3}})}_{3}}N\]
Primary amine may be obtained in a good yield by using a excess of ammonia.
(ii) Methods yielding primary amines
(a) Reduction of nitro compounds
\[R-N{{O}_{2}}+6[H]\underset{{Zn}/{HCl\text{ or }Ni\text{ or }LiAl{{H}_{4}}}\;}{\mathop{\xrightarrow{\,\,\,\,\,\,\,{Sn}/{HCl\text{ or}}\;\,\,\,\,\,\,}}}\,RN{{H}_{2}}+2{{H}_{2}}O\]
\[{{C}_{2}}{{H}_{5}}-N{{O}_{2}}+6[H]\to {{C}_{2}}{{H}_{5}}N{{H}_{2}}+2{{H}_{2}}O\]
(b) Reduction of nitriles (Mendius reaction)
\[R-C\equiv N+4[H]\to R-C{{H}_{2}}N{{H}_{2}}\]
\[\underset{\text{Methyl cyanide}}{\mathop{C{{H}_{3}}C\equiv N}}\,+4[H]\to \underset{\text{Ethylamine}}{\mathop{C{{H}_{3}}-C{{H}_{2}}N{{H}_{2}}}}\,\]
The start can be made from alcohol or alkyl halide.
\[\underset{\text{Alcohol}}{\mathop{R-OH}}\,\xrightarrow{SOC{{l}_{2}}}\underset{\text{Alkyl chloride}}{\mathop{R-Cl}}\,\xrightarrow{KCN}\underset{\text{Alkyl nitrile}}{\mathop{R-CN}}\,\underset{Na+{{C}_{2}}{{H}_{5}}OH}{\mathop{\xrightarrow{LiAl{{H}_{4}}or}}}\,\underset{\text{Primary amine}}{\mathop{RC{{H}_{2}}N{{H}_{2}}}}\,\]\[\underset{\text{Alkyl nitrile}}{\mathop{R-CN}}\,\underset{Na+{{C}_{2}}{{H}_{5}}OH}{\mathop{\xrightarrow{LiAl{{H}_{4}}or}}}\,\underset{\text{Primary amine}}{\mathop{RC{{H}_{2}}N{{H}_{2}}}}\,\]
This sequence gives an amine containing one more carbon atom than alcohol.
(c) By reduction of amides with \[LiAl{{H}_{4}}\]
\[RCON{{H}_{2}}\xrightarrow{LiAl{{H}_{4}}}RC{{H}_{2}}N{{H}_{2}}\]
\[\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,\xrightarrow{LiAl{{H}_{4}}}\underset{\text{Ethylamine}}{\mathop{C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}}}\,\]
(d) By reduction of oximes : The start can be made from an aldehyde or ketone.
\[\underset{\text{Aldehyde}}{\mathop{RCHO}}\,\xrightarrow{{{H}_{2}}NOH}\underset{\text{Oxime}}{\mathop{RCH=NOH}}\,\underset{{or{{H}_{2}}}/{Ni}\;}{\mathop{\xrightarrow{LiAl{{H}_{4}}}}}\,\underset{\text{Primary amine}}{\mathop{RC{{H}_{2}}N{{H}_{2}}}}\,\]
Amines are classified as primary, secondary or tertiary depending on the number of alkyl groups attached to nitrogen atom.
The characteristic groups in primary, secondary and tertiary amines are: \[\underset{\text{(amino)}}{\mathop{N{{H}_{2}}}}\,\]; \[\underset{\text{(imino)}}{\overset{|}{\mathop{NH}}}\,\]; \[\underset{(tert-\text{nitrogen)}}{\mathop{\underset{|}{\overset{|}{\mathop{-N\,\,\,}}}\,}}\,\]
In addition to above amines, tetra-alkyl derivatives similar to ammonium salts also exist which are called quaternary ammonium compounds.
\[N{{H}_{4}}I\]; \[\underset{\begin{smallmatrix} \,\,\,\,\,\,\text{Quaternary} \\ \text{ammonium }\,\text{iodide} \end{smallmatrix}}{\mathop{{{R}_{4}}NI}}\,\]; \[\underset{\begin{smallmatrix} \,\,\,\,\text{Tetramethyl} \\ \text{ammonium iodide} \end{smallmatrix}}{\mathop{{{(C{{H}_{3}})}_{4}}NI}}\,\] or \[\underset{\begin{smallmatrix} \,\,\,\,\text{Tetra-alkyl} \\ \text{ammonium salt} \end{smallmatrix}}{\mathop{{{\left[ R-\underset{\underset{R}{\mathop{|}}\,}{\overset{\overset{R}{\mathop{|}}\,}{\mathop{N}}}\,-R \right]}^{+}}}}\,{{X}^{-}}\]
(1) Simple and mixed amines : Secondary and tertiary amines may be classified as simple or mixed amines according as all the alkyl or aryl groups attached to the nitrogen atom are same or different. For example,
Simple amines : \[\underset{\text{Dimethylamine}}{\mathop{{{(C{{H}_{3}})}_{2}}NH}}\,\]; \[\underset{\text{Triethylamine}}{\mathop{{{(C{{H}_{3}}C{{H}_{2}})}_{3}}N}}\,\]
Mixed amines : \[\underset{\text{Ethylmethylamine}}{\mathop{{{C}_{2}}{{H}_{5}}-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{N}}\,H}}\,}}\,\]; \[\underset{\text{Methylaniline}}{\mathop{{{C}_{6}}{{H}_{5}}-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{N}}\,H}}\,}}\,\]
The aliphatic amines have pyramidal shape with one electron pair. In amines, N undergoes hybridisation.
(2) General methods of preparation
(i) Methods yielding mixture of amines (Primary, secondary and tertiary)
(a) Hofmann's method :The mixture of amines (\[{{1}^{o}},\,\,{{2}^{o}}\] and \[{{3}^{o}}\]) is formed by the alkylation of ammonia with alkyl halides.
\[\underset{\text{Methyliodi}\text{de}}{\mathop{C{{H}_{3}}I}}\,+N{{H}_{3}}\to \underset{\begin{smallmatrix} \text{Methylamine} \\ \,\,\,\,\,\text{(1}{}^\circ \text{)} \end{smallmatrix}}{\mathop{C{{H}_{3}}N{{H}_{2}}}}\,\xrightarrow{C{{H}_{3}}I}(\underset{\begin{smallmatrix} \text{Dimethylamine} \\ \,\,\,\,\,\,\,\text{(2}{}^\circ \text{)} \end{smallmatrix}}{\mathop{C{{H}_{3}}{{)}_{2}}NH}}\,\]
\[\xrightarrow{C{{H}_{3}}I}\underset{\begin{smallmatrix} \text{Trimethylamine} \\ \,\,\,\,\,\,\,\,\,\,\,\text{(3}{}^\circ \text{)} \end{smallmatrix}}{\mathop{{{(C{{H}_{3}})}_{3}}N}}\,\xrightarrow{C{{H}_{3}}I}\underset{\begin{smallmatrix} \,\,\,\,\text{Tetramethyl} \\ \text{ammonium iodide} \end{smallmatrix}}{\mathop{{{(C{{H}_{3}})}_{4}}NI}}\,\]
The primary amine may be obtained in a good yield by using a large excess of ammonia. The process is also termed as ammonolysis of alkyl halides. It is a nucleophilic substitution reaction.
(b) Ammonolysis of alcohols :
\[C{{H}_{3}}OH+N{{H}_{3}}\underset{350{}^\circ C}{\mathop{\xrightarrow{A{{l}_{2}}{{O}_{3}}}}}\,C{{H}_{3}}N{{H}_{2}}\]\[\xrightarrow{C{{H}_{3}}OH}{{(C{{H}_{3}})}_{2}}NH\xrightarrow{C{{H}_{3}}OH}{{(C{{H}_{3}})}_{3}}N\]
Primary amine may be obtained in a good yield by using a excess of ammonia.
(ii) Methods yielding primary amines
(a) Reduction of nitro compounds
\[R-N{{O}_{2}}+6[H]\underset{{Zn}/{HCl\text{ or }Ni\text{ or }LiAl{{H}_{4}}}\;}{\mathop{\xrightarrow{\,\,\,\,\,\,\,{Sn}/{HCl\text{ or}}\;\,\,\,\,\,\,}}}\,RN{{H}_{2}}+2{{H}_{2}}O\]
\[{{C}_{2}}{{H}_{5}}-N{{O}_{2}}+6[H]\to {{C}_{2}}{{H}_{5}}N{{H}_{2}}+2{{H}_{2}}O\]
(b) Reduction of nitriles (Mendius reaction)
\[R-C\equiv N+4[H]\to R-C{{H}_{2}}N{{H}_{2}}\]
\[\underset{\text{Methyl cyanide}}{\mathop{C{{H}_{3}}C\equiv N}}\,+4[H]\to \underset{\text{Ethylamine}}{\mathop{C{{H}_{3}}-C{{H}_{2}}N{{H}_{2}}}}\,\]
The start can be made from alcohol or alkyl halide.
\[\underset{\text{Alcohol}}{\mathop{R-OH}}\,\xrightarrow{SOC{{l}_{2}}}\underset{\text{Alkyl chloride}}{\mathop{R-Cl}}\,\xrightarrow{KCN}\underset{\text{Alkyl nitrile}}{\mathop{R-CN}}\,\underset{Na+{{C}_{2}}{{H}_{5}}OH}{\mathop{\xrightarrow{LiAl{{H}_{4}}or}}}\,\underset{\text{Primary amine}}{\mathop{RC{{H}_{2}}N{{H}_{2}}}}\,\]\[\underset{\text{Alkyl nitrile}}{\mathop{R-CN}}\,\underset{Na+{{C}_{2}}{{H}_{5}}OH}{\mathop{\xrightarrow{LiAl{{H}_{4}}or}}}\,\underset{\text{Primary amine}}{\mathop{RC{{H}_{2}}N{{H}_{2}}}}\,\]
This sequence gives an amine containing one more carbon atom than alcohol.
(c) By reduction of amides with \[LiAl{{H}_{4}}\]
\[RCON{{H}_{2}}\xrightarrow{LiAl{{H}_{4}}}RC{{H}_{2}}N{{H}_{2}}\]
\[\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,\xrightarrow{LiAl{{H}_{4}}}\underset{\text{Ethylamine}}{\mathop{C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}}}\,\]
(d) By reduction of oximes : The start can be made from an aldehyde or ketone.
\[\underset{\text{Aldehyde}}{\mathop{RCHO}}\,\xrightarrow{{{H}_{2}}NOH}\underset{\text{Oxime}}{\mathop{RCH=NOH}}\,\underset{{or{{H}_{2}}}/{Ni}\;}{\mathop{\xrightarrow{LiAl{{H}_{4}}}}}\,\underset{\text{Primary amine}}{\mathop{RC{{H}_{2}}N{{H}_{2}}}}\,\]
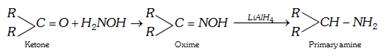 (e) Hofmann's bromamide reaction or degradation (Laboratory method) : By this method the amide \[(CON{{H}_{2}})\] group is converted into primary amino \[(\text{ }N{{H}_{2}})\] group.
\[R-\underset{\text{Amide}}{\mathop{CO-N{{H}_{2}}}}\,+B{{r}_{2}}+4KOH\to \underset{\text{Pri-amine}}{\mathop{R-N{{H}_{2}}}}\,+2KBr+{{K}_{2}}C{{O}_{3}}+2{{H}_{2}}O\]
This is the most convenient method for preparing primary amines.
This method gives an amine containing one carbon atom less than amide.
(f) Gabriel phthalimide synthesis : This method involves the following three steps.
(e) Hofmann's bromamide reaction or degradation (Laboratory method) : By this method the amide \[(CON{{H}_{2}})\] group is converted into primary amino \[(\text{ }N{{H}_{2}})\] group.
\[R-\underset{\text{Amide}}{\mathop{CO-N{{H}_{2}}}}\,+B{{r}_{2}}+4KOH\to \underset{\text{Pri-amine}}{\mathop{R-N{{H}_{2}}}}\,+2KBr+{{K}_{2}}C{{O}_{3}}+2{{H}_{2}}O\]
This is the most convenient method for preparing primary amines.
This method gives an amine containing one carbon atom less than amide.
(f) Gabriel phthalimide synthesis : This method involves the following three steps.
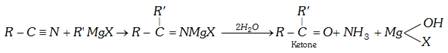
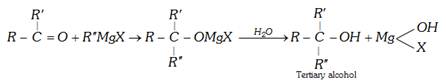 (d) Alcohololysis :
\[\underset{\begin{smallmatrix} \,\text{Alkyl} \\ \text{cyanide} \end{smallmatrix}}{\mathop{RCN}}\,+\underset{\text{Alcohol}}{\mathop{{R}'OH}}\,+HCl\to \underset{\text{imido ester}}{\mathop{\left[ \overset{\,\,\,\overset{+}{\mathop{N}}\,{{H}_{2}}}{\mathop{R-\overset{|\,|}{\mathop{C}}\,-O{R}'}}\, \right]}}\,\,\,C{{l}^{-}}\]\[\xrightarrow{{{H}_{2}}O}\underset{\text{Ester}}{\mathop{RCOO{R}'}}\,\ +N{{H}_{4}}Cl\]
(iv) Uses : Alkyl cyanides are important intermediates in the organic synthesis of a large number of compounds like acids, amides, esters, amines etc.
(2) Alkyl Isocyanides
(i) Methods of preparation
(a) From alkyl halides :
\[\underset{\text{Alkyl halide}}{\mathop{R-X}}\,+AgCN\to \underset{\begin{smallmatrix} \,\,\text{Isocyanide} \\ \,\,\text{(Isonitrile)} \\ \text{Main product} \end{smallmatrix}}{\mathop{RNC}}\,+\underset{\begin{smallmatrix} \,\,\,\,\text{Cyanide} \\ \,\,\,\,\text{(Nitrile)} \\ \text{Minor pro}\text{duct} \end{smallmatrix}}{\mathop{RCN}}\,\] \[\underset{\text{Methyl chloride}}{\mathop{C{{H}_{3}}Cl}}\,+AgCN\to \underset{\begin{smallmatrix} \text{Methyl isocyanide} \\ \,\,\text{(Main product)} \end{smallmatrix}}{\mathop{C{{H}_{3}}NC}}\,+C{{H}_{3}}CN\]
(b) From primary amines (Carbylamine reaction) :
\[\underset{\text{Primary amine}}{\mathop{RN{{H}_{2}}}}\,+\underset{\text{Chloroform}}{\mathop{CHC{{l}_{3}}}}\,+3KOH\to \underset{\text{Isocyanide}}{\mathop{RNC}}\,+3KCl+3{{H}_{2}}O\]
(c) From N-alkyl more...
(d) Alcohololysis :
\[\underset{\begin{smallmatrix} \,\text{Alkyl} \\ \text{cyanide} \end{smallmatrix}}{\mathop{RCN}}\,+\underset{\text{Alcohol}}{\mathop{{R}'OH}}\,+HCl\to \underset{\text{imido ester}}{\mathop{\left[ \overset{\,\,\,\overset{+}{\mathop{N}}\,{{H}_{2}}}{\mathop{R-\overset{|\,|}{\mathop{C}}\,-O{R}'}}\, \right]}}\,\,\,C{{l}^{-}}\]\[\xrightarrow{{{H}_{2}}O}\underset{\text{Ester}}{\mathop{RCOO{R}'}}\,\ +N{{H}_{4}}Cl\]
(iv) Uses : Alkyl cyanides are important intermediates in the organic synthesis of a large number of compounds like acids, amides, esters, amines etc.
(2) Alkyl Isocyanides
(i) Methods of preparation
(a) From alkyl halides :
\[\underset{\text{Alkyl halide}}{\mathop{R-X}}\,+AgCN\to \underset{\begin{smallmatrix} \,\,\text{Isocyanide} \\ \,\,\text{(Isonitrile)} \\ \text{Main product} \end{smallmatrix}}{\mathop{RNC}}\,+\underset{\begin{smallmatrix} \,\,\,\,\text{Cyanide} \\ \,\,\,\,\text{(Nitrile)} \\ \text{Minor pro}\text{duct} \end{smallmatrix}}{\mathop{RCN}}\,\] \[\underset{\text{Methyl chloride}}{\mathop{C{{H}_{3}}Cl}}\,+AgCN\to \underset{\begin{smallmatrix} \text{Methyl isocyanide} \\ \,\,\text{(Main product)} \end{smallmatrix}}{\mathop{C{{H}_{3}}NC}}\,+C{{H}_{3}}CN\]
(b) From primary amines (Carbylamine reaction) :
\[\underset{\text{Primary amine}}{\mathop{RN{{H}_{2}}}}\,+\underset{\text{Chloroform}}{\mathop{CHC{{l}_{3}}}}\,+3KOH\to \underset{\text{Isocyanide}}{\mathop{RNC}}\,+3KCl+3{{H}_{2}}O\]
(c) From N-alkyl more... 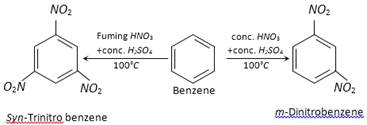 (b) Temperature of nitration : For example,
(b) Temperature of nitration : For example,
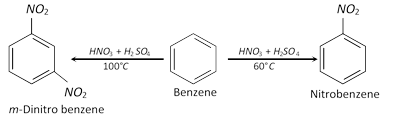 (c) Nature of the compound to be nitrated : Presence of electron-releasing group like \[OH,N{{H}_{2}},~C{{H}_{3}},OR,\] etc., in the nucleus facilitates nitration. Thus aromatic compounds bearing these groups (i.e. phenol, aniline, toluene, etc.) can be nitrated readily as compared to benzene. Thus benzene is not affected by dilute HNO3 while phenol, aniline and toluene forms the corresponding ortho- and para-nitro compounds.
(c) Nature of the compound to be nitrated : Presence of electron-releasing group like \[OH,N{{H}_{2}},~C{{H}_{3}},OR,\] etc., in the nucleus facilitates nitration. Thus aromatic compounds bearing these groups (i.e. phenol, aniline, toluene, etc.) can be nitrated readily as compared to benzene. Thus benzene is not affected by dilute HNO3 while phenol, aniline and toluene forms the corresponding ortho- and para-nitro compounds.

 On the other hand, nitration of aromatic compounds having electron withdrawing groups like \[N{{O}_{2}},S{{O}_{3}}H\] requires powerful nitrating agent (like fuming \[HN{{O}_{3}}+\] conc. \[{{H}_{2}}S{{O}_{4}}\]) and a high temperature.
(ii) Indirect method : The aromatic nitro compounds which can not be prepared by direct method may be prepared from the corresponding amino compound.
On the other hand, nitration of aromatic compounds having electron withdrawing groups like \[N{{O}_{2}},S{{O}_{3}}H\] requires powerful nitrating agent (like fuming \[HN{{O}_{3}}+\] conc. \[{{H}_{2}}S{{O}_{4}}\]) and a high temperature.
(ii) Indirect method : The aromatic nitro compounds which can not be prepared by direct method may be prepared from the corresponding amino compound.
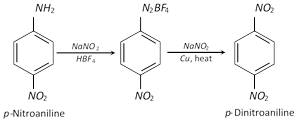 (2) Physical properties
(i) Aromatic nitro compounds are insoluble in water but soluble in organic solvents.
(ii) They are either pale yellow liquids or solids having distinct smells. For example, nitro benzene (oil of Mirabane) is a pale yellow liquid having a smell of bitter almonds.
(3) Chemical properties
(i) Resonance in nitrobenzene imparts a partial double bond character to the bond between carbon of benzene nucleus and nitrogen of the \[N{{O}_{2}}\] group with the result the \[N{{O}_{2}}\] group is firmly bonded to the ring and therefore cannot be replaced other groups, i.e., it is very inert.
(2) Physical properties
(i) Aromatic nitro compounds are insoluble in water but soluble in organic solvents.
(ii) They are either pale yellow liquids or solids having distinct smells. For example, nitro benzene (oil of Mirabane) is a pale yellow liquid having a smell of bitter almonds.
(3) Chemical properties
(i) Resonance in nitrobenzene imparts a partial double bond character to the bond between carbon of benzene nucleus and nitrogen of the \[N{{O}_{2}}\] group with the result the \[N{{O}_{2}}\] group is firmly bonded to the ring and therefore cannot be replaced other groups, i.e., it is very inert.
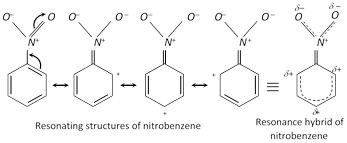 (ii) Displacement of the \[N{{O}_{2}}\] group : Although \[N{{O}_{2}}\] group of nitrobenzene cannot be replaced by other groups, but if a second \[N{{O}_{2}}\] group is present on the benzene ring of nitrobenzene in the \[o-\] or \[p-\]position, it can be replaced by a nucleophile. For example,
(ii) Displacement of the \[N{{O}_{2}}\] group : Although \[N{{O}_{2}}\] group of nitrobenzene cannot be replaced by other groups, but if a second \[N{{O}_{2}}\] group is present on the benzene ring of nitrobenzene in the \[o-\] or \[p-\]position, it can be replaced by a nucleophile. For example,
 (iii) Reduction : Aromatic nitro compounds can be reduced to a variety of product as shown below in the case of nitrobenzene.
\[\underset{\text{Nitrobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}N{{O}_{2}}}}\,\to \underset{\text{Nitrosobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}NO}}\,\to \underset{\text{Phenylhydroxylamine}}{\mathop{{{C}_{6}}{{H}_{5}}NHOH}}\,\to \underset{\text{Aniline}}{\mathop{{{C}_{6}}{{H}_{5}}N{{H}_{2}}}}\,\]
The nature of the final product depends mainly on the nature (acidic, basic or neutral) of the reduction medium and the nature of the reducing agent.
(a) Reduction in acidic medium
(iii) Reduction : Aromatic nitro compounds can be reduced to a variety of product as shown below in the case of nitrobenzene.
\[\underset{\text{Nitrobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}N{{O}_{2}}}}\,\to \underset{\text{Nitrosobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}NO}}\,\to \underset{\text{Phenylhydroxylamine}}{\mathop{{{C}_{6}}{{H}_{5}}NHOH}}\,\to \underset{\text{Aniline}}{\mathop{{{C}_{6}}{{H}_{5}}N{{H}_{2}}}}\,\]
The nature of the final product depends mainly on the nature (acidic, basic or neutral) of the reduction medium and the nature of the reducing agent.
(a) Reduction in acidic medium
 Reduction of dinitrobenzene with ammonium sulphide reduces only one more...
Reduction of dinitrobenzene with ammonium sulphide reduces only one more...  (ii) General methods of preparation
(a) By heating an alkyl halide with aqueous alcoholic solution of silver nitrite
\[{{C}_{2}}{{H}_{5}}Br+AgN{{O}_{2}}\to {{C}_{2}}{{H}_{5}}N{{O}_{2}}+AgBr\]
Some quantity of alkyl nitrite is also formed in this reaction. It can be removed by fractional distillation since alkyl nitrites have much lower boiling points as compared to nitro alkanes.
(b) By the direct nitration of paraffins (Vapour phase nitration)
\[C{{H}_{3}}C{{H}_{3}}+HON{{O}_{2}}(\text{fuming})\,\xrightarrow{400{}^\circ C}C{{H}_{3}}C{{H}_{2}}N{{O}_{2}}+{{H}_{2}}O\]
With higher alkanes, a mixture of different nitro alkanes is formed which can be separated by fractional distillation.
(c) By the action of sodium nitrite on a-halo carboxylic acids
\[\underset{\alpha \text{--Chloro acetic acid}}{\mathop{C{{H}_{2}}ClOOH}}\,\underset{-NaCl}{\mathop{\xrightarrow{NaN{{O}_{2}}}}}\,\underset{\alpha \text{Nitro acetic acid}}{\mathop{C{{H}_{2}}N{{O}_{2}}COOH}}\,\]\[\xrightarrow{\text{heat}}\underset{\text{Nitro methane}}{\mathop{C{{H}_{3}}N{{O}_{2}}}}\,\ +C{{O}_{2}}\]
(d) By the hydrolysis more...
(ii) General methods of preparation
(a) By heating an alkyl halide with aqueous alcoholic solution of silver nitrite
\[{{C}_{2}}{{H}_{5}}Br+AgN{{O}_{2}}\to {{C}_{2}}{{H}_{5}}N{{O}_{2}}+AgBr\]
Some quantity of alkyl nitrite is also formed in this reaction. It can be removed by fractional distillation since alkyl nitrites have much lower boiling points as compared to nitro alkanes.
(b) By the direct nitration of paraffins (Vapour phase nitration)
\[C{{H}_{3}}C{{H}_{3}}+HON{{O}_{2}}(\text{fuming})\,\xrightarrow{400{}^\circ C}C{{H}_{3}}C{{H}_{2}}N{{O}_{2}}+{{H}_{2}}O\]
With higher alkanes, a mixture of different nitro alkanes is formed which can be separated by fractional distillation.
(c) By the action of sodium nitrite on a-halo carboxylic acids
\[\underset{\alpha \text{--Chloro acetic acid}}{\mathop{C{{H}_{2}}ClOOH}}\,\underset{-NaCl}{\mathop{\xrightarrow{NaN{{O}_{2}}}}}\,\underset{\alpha \text{Nitro acetic acid}}{\mathop{C{{H}_{2}}N{{O}_{2}}COOH}}\,\]\[\xrightarrow{\text{heat}}\underset{\text{Nitro methane}}{\mathop{C{{H}_{3}}N{{O}_{2}}}}\,\ +C{{O}_{2}}\]
(d) By the hydrolysis more... 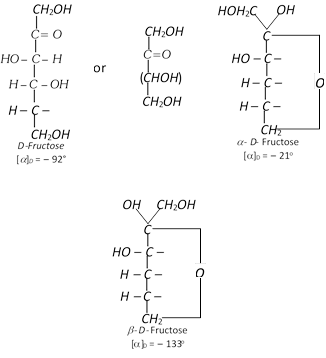 Comparison between glucose and fructose
Comparison between glucose and fructose
| Property | Glucose | Fructose | ||||||||||||||
| Molecular formula | \[{{C}_{6}}{{H}_{12}}{{O}_{6}}\] | \[{{C}_{6}}{{H}_{12}}{{O}_{6}}\] | ||||||||||||||
| Nature | Polyhydroxy aldehyde. | Polyhydroxy ketone | ||||||||||||||
| Melting point | \[146{}^\circ C\] |
| Group replacing \[-OH\] | Name | Structure |
| \[(X=F,\,Cl,\,Br,\,I)\] | Acyl halide | \[R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-X\] |
| \[-N{{H}_{2}}\] | Amide | \[R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-N{{H}_{2}}\] |
| \[-O{R}'\] | ester | \[\underset{\text{(}{R}'\,\text{may be }R\text{)}}{\mathop{R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-O{R}'}}\,\] |
| \[-OOCR\] | anhydride | \[R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-O-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-R\] |
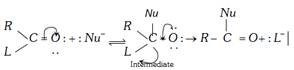 \[(L=X,\,N{{H}_{2}},\,O-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-R\,\text{or}\,OR)\]
The relative reactivities of various acyl compounds have been found to be in the following order:
\[(L=X,\,N{{H}_{2}},\,O-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-R\,\text{or}\,OR)\]
The relative reactivities of various acyl compounds have been found to be in the following order:
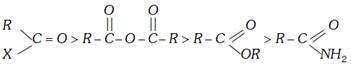 Out of acid halides, the acid chlorides are more important ones.
The overall order of reactivity can be accounted for in terms of the following three factors:
(i) Basicity of the leaving group (ii) Resonance effects and (iii) Inductive effects.
(i) Basicity of the leaving group : Weaker bases are good leaving groups. Hence, the acyl derivatives more...
Out of acid halides, the acid chlorides are more important ones.
The overall order of reactivity can be accounted for in terms of the following three factors:
(i) Basicity of the leaving group (ii) Resonance effects and (iii) Inductive effects.
(i) Basicity of the leaving group : Weaker bases are good leaving groups. Hence, the acyl derivatives more... 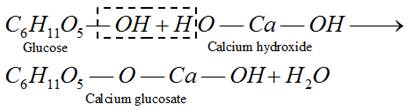
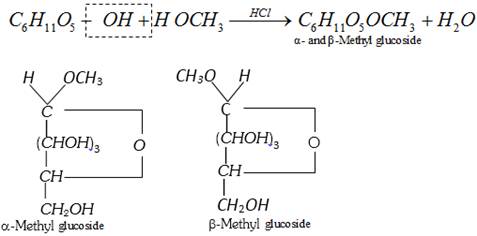 This reaction shows the presence of ring structure in glucose.
(ii) Reactions of carbonyl group (Aldehydic group)
(a) Reduction
\[\underset{\text{Glucose}}{\mathop{C{{H}_{2}}OH{{(CHOH)}_{4}}CHO}}\,+2H\underset{{{H}_{2}}O}{\mathop{\xrightarrow{Na-Hg}}}\,\underset{\text{Sorbitol}}{\mathop{C{{H}_{2}}OH{{(CHOH)}_{4}}C{{H}_{2}}OH}}\,\] On prolonged heating with concentrated HI and red phosphorus at \[{{110}^{o}}C\], glucose forms a mixture of 2-iodohexane and n-hexane.
(b) Oxidation
This reaction shows the presence of ring structure in glucose.
(ii) Reactions of carbonyl group (Aldehydic group)
(a) Reduction
\[\underset{\text{Glucose}}{\mathop{C{{H}_{2}}OH{{(CHOH)}_{4}}CHO}}\,+2H\underset{{{H}_{2}}O}{\mathop{\xrightarrow{Na-Hg}}}\,\underset{\text{Sorbitol}}{\mathop{C{{H}_{2}}OH{{(CHOH)}_{4}}C{{H}_{2}}OH}}\,\] On prolonged heating with concentrated HI and red phosphorus at \[{{110}^{o}}C\], glucose forms a mixture of 2-iodohexane and n-hexane.
(b) Oxidation