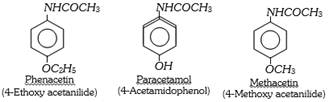
 The derivatives of p-aminophenol are used as antipyretic. The main limitation of these derivatives is that they may act on red blood cells and thus, they may be harmful in moderate doses. The important derivatives are,
The derivatives of p-aminophenol are used as antipyretic. The main limitation of these derivatives is that they may act on red blood cells and thus, they may be harmful in moderate doses. The important derivatives are,
 Phenyl butazone is a pyrazolone derivative. Its structure is,
Phenyl butazone is a pyrazolone derivative. Its structure is,
 It is highly toxic and hence not considered as a safe drug. Oxyphenyl butazone is less toxic and is used in place of phenyl butazone.
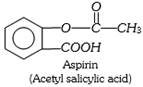
(2) Analgesics : Drugs which relieve or decrease pain are termed analgesics. These are of two types,
(i) Narcotics : These are mainly opium and its products such more...
It is highly toxic and hence not considered as a safe drug. Oxyphenyl butazone is less toxic and is used in place of phenyl butazone.
(2) Analgesics : Drugs which relieve or decrease pain are termed analgesics. These are of two types,
(i) Narcotics : These are mainly opium and its products such more... 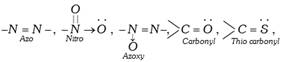
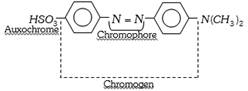
 [Chromophore-Greek word, Chroma = colour, Phorein = to bear].
The presence of chromophore is not necessarily sufficient for colour. To make a substance coloured, the chromophore has to be conjugated with an extensive system of alternate single and double bonds as exists in aromatic compounds.
The chromophore part of the coloured substance (dye) absorbs some wavelengths from white light and reflects back the complementary colour. A coloured compound having a chromophore is known as chromogen.
(ii) Certain groups, while not producing colour themselves, when present along with a chromophore in an organic substance, intensify the colour. Such colour assisting groups are called auxochromes (Greek word, Auxanien = to increase; Chrome = colour), i.e. they make the colour deep and fast and fix the dye to the fabric. The auxochromes are acidic or basic functional groups. The important auxochromes are,
Acidic : \[\underset{\text{Hydroxy}}{\mathop{-OH}}\,\], \[\underset{\text{Sulphonic}}{\mathop{-S{{O}_{3}}H}}\,\], \[\underset{\text{Carboxylic}}{\mathop{-COOH}}\,\]
Basic : \[\underset{\text{Amino}}{\mathop{-N{{H}_{2}}}}\,\], \[\underset{\text{Alkylamino}}{\mathop{-NHR}}\,\], \[\underset{\text{Dialkylamino}}{\mathop{-N{{R}_{2}}}}\,\]
Example :
[Chromophore-Greek word, Chroma = colour, Phorein = to bear].
The presence of chromophore is not necessarily sufficient for colour. To make a substance coloured, the chromophore has to be conjugated with an extensive system of alternate single and double bonds as exists in aromatic compounds.
The chromophore part of the coloured substance (dye) absorbs some wavelengths from white light and reflects back the complementary colour. A coloured compound having a chromophore is known as chromogen.
(ii) Certain groups, while not producing colour themselves, when present along with a chromophore in an organic substance, intensify the colour. Such colour assisting groups are called auxochromes (Greek word, Auxanien = to increase; Chrome = colour), i.e. they make the colour deep and fast and fix the dye to the fabric. The auxochromes are acidic or basic functional groups. The important auxochromes are,
Acidic : \[\underset{\text{Hydroxy}}{\mathop{-OH}}\,\], \[\underset{\text{Sulphonic}}{\mathop{-S{{O}_{3}}H}}\,\], \[\underset{\text{Carboxylic}}{\mathop{-COOH}}\,\]
Basic : \[\underset{\text{Amino}}{\mathop{-N{{H}_{2}}}}\,\], \[\underset{\text{Alkylamino}}{\mathop{-NHR}}\,\], \[\underset{\text{Dialkylamino}}{\mathop{-N{{R}_{2}}}}\,\]
Example :
 However, Otto witt chromophore-Auxochrome concept fails to explain the colour of certain dye stuffs like indigo.
(2) Classification of Dyes : Dyes are classified to their chemical constitution or by their application to the fibre.
(i) Classification of dyes according to their chemical structure
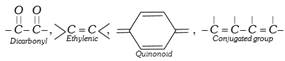
(a) Nitro and Nitroso dyes : These dyes contain nitro or nitroso groups as the chromophores and \[-OH\] as auxochrome. A few example are,
However, Otto witt chromophore-Auxochrome concept fails to explain the colour of certain dye stuffs like indigo.
(2) Classification of Dyes : Dyes are classified to their chemical constitution or by their application to the fibre.
(i) Classification of dyes according to their chemical structure
(a) Nitro and Nitroso dyes : These dyes contain nitro or nitroso groups as the chromophores and \[-OH\] as auxochrome. A few example are,
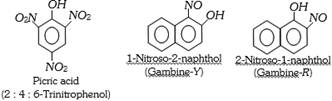
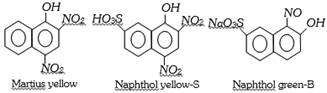 (b) Azo dyes : The azo dyes contain one or more azo groups – N=N–, as the chromophore. Azo dyes constitute the largest and most important group of synthetic dyes. These can be prepared by diazotising an aromatic amine and more...
(b) Azo dyes : The azo dyes contain one or more azo groups – N=N–, as the chromophore. Azo dyes constitute the largest and most important group of synthetic dyes. These can be prepared by diazotising an aromatic amine and more...  Aromatic compounds : These compounds consist of at least one benzene ring, i.e., a six-membered carbocyclic ring having alternate single and double bonds. Generally, these compounds have some fragrant odour and hence, named as aromatic (Greek word aroma meaning sweet smell).
Aromatic compounds : These compounds consist of at least one benzene ring, i.e., a six-membered carbocyclic ring having alternate single and double bonds. Generally, these compounds have some fragrant odour and hence, named as aromatic (Greek word aroma meaning sweet smell).
 These are also called benzenoid aromatics.
Non-benzenoid aromatics : There are aromatic compounds, which have structural units different from benzenoid type and are known as Non-benzenoid aromatics e.g. Tropolone, azulene etc.
These are also called benzenoid aromatics.
Non-benzenoid aromatics : There are aromatic compounds, which have structural units different from benzenoid type and are known as Non-benzenoid aromatics e.g. Tropolone, azulene etc.
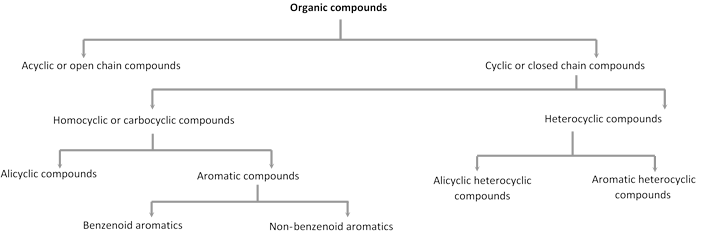
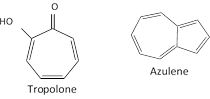 (b) Heterocyclic compounds : Cyclic compounds containing one or more hetero atoms (e.g. O, N, S etc.) in the ring are called heterocyclic compounds. These are of two types :
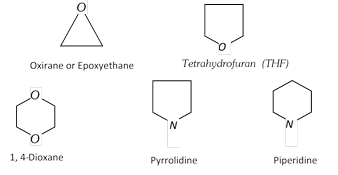
Alicyclic heterocyclic compounds : Heterocyclic compounds which resemble aliphatic compounds in their properties are called Alicyclic heterocyclic compounds. For example,
(b) Heterocyclic compounds : Cyclic compounds containing one or more hetero atoms (e.g. O, N, S etc.) in the ring are called heterocyclic compounds. These are of two types :
Alicyclic heterocyclic compounds : Heterocyclic compounds which resemble aliphatic compounds in their properties are called Alicyclic heterocyclic compounds. For example,
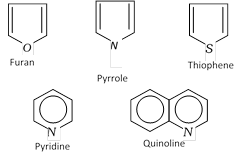
 Aromatic heterocyclic compounds : Heterocyclic compounds which resemble benzene and other aromatic compounds in most of their properties are called Aromatic heterocyclic compounds. For example,
Aromatic heterocyclic compounds : Heterocyclic compounds which resemble benzene and other aromatic compounds in most of their properties are called Aromatic heterocyclic compounds. For example,
 (2) Classification based on functional groups : A functional group is an atom or group of atoms in a molecule that gives the molecule its characteristic chemical properties. Double and triple bonds are also considered more...
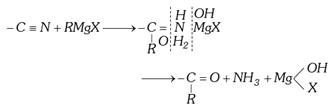
(2) Classification based on functional groups : A functional group is an atom or group of atoms in a molecule that gives the molecule its characteristic chemical properties. Double and triple bonds are also considered more... 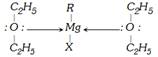 Grignard reagents are never isolated in free sate on account of their explosive nature.
For given alkyl radical the ease of formation of a grignard reagent is, Iodide > Bromide > Chloride Usually alkyl bromides are used.
For a given halogen, the ease of formation of grignard reagent is,\[C{{H}_{3}}X>{{C}_{2}}{{H}_{5}}X>{{C}_{3}}{{H}_{7}}X..........\]
Since tertiary alkyl iodides eliminate HI to form an alkene, tertiary alkyl chlorides are used in place of tertiary alkyl iodides.
Grignard reagents are never isolated in free sate on account of their explosive nature.
For given alkyl radical the ease of formation of a grignard reagent is, Iodide > Bromide > Chloride Usually alkyl bromides are used.
For a given halogen, the ease of formation of grignard reagent is,\[C{{H}_{3}}X>{{C}_{2}}{{H}_{5}}X>{{C}_{3}}{{H}_{7}}X..........\]
Since tertiary alkyl iodides eliminate HI to form an alkene, tertiary alkyl chlorides are used in place of tertiary alkyl iodides.
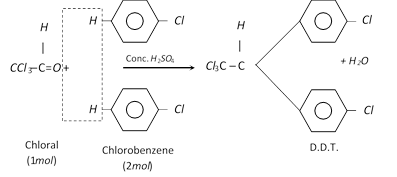 Properties and uses of D.D.T.
(i) D.D.T. is almost insoluble in water but it is moderately soluble in polar solvents.
(ii) D.D.T. is a powerful insecticide. It is widely used as an insecticide for killing mosquitoes and other insects.
Side Effects of D.D.T. : more...
Properties and uses of D.D.T.
(i) D.D.T. is almost insoluble in water but it is moderately soluble in polar solvents.
(ii) D.D.T. is a powerful insecticide. It is widely used as an insecticide for killing mosquitoes and other insects.
Side Effects of D.D.T. : more...  (2) Methods of preparation
(i) By direct halogination of benzene ring
(2) Methods of preparation
(i) By direct halogination of benzene ring
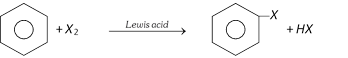 Lewis acid \[=Fe{{X}_{3}},Al{{X}_{3}},\,Tl{{(OAC)}_{3}}\]; \[{{X}_{2}}=C{{l}_{2}},B{{r}_{2}}\]
(ii) From diazonium salts
Lewis acid \[=Fe{{X}_{3}},Al{{X}_{3}},\,Tl{{(OAC)}_{3}}\]; \[{{X}_{2}}=C{{l}_{2}},B{{r}_{2}}\]
(ii) From diazonium salts
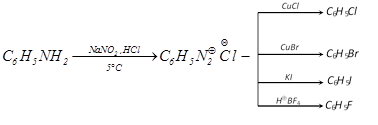 (iii) Hunsdiecker reaction :
\[{{C}_{6}}{{H}_{5}}CO{{O}^{-}}A{{g}^{+}}\xrightarrow{B{{r}_{2}}}{{C}_{6}}{{H}_{5}}Br+C{{O}_{2}}+AgBr\]
(iv) From Aryl thalium compound :
\[ArH+Tl{{(OOCC{{F}_{3}})}_{3}}\xrightarrow[-C{{F}_{3}}C{{O}_{2}}H]{}\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\text{Aryl thallium trifluoroacetate}}{\mathop{ArTl{{(OOC{{F}_{3}})}_{2}}\underset{\Delta }{\mathop{\xrightarrow{KI}}}\,ArI}}\,\] \[\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\text{Aryl thallium trifluoroacetate}}{\mathop{ArTl{{(OOC{{F}_{3}})}_{2}}\underset{\Delta }{\mathop{\xrightarrow{KI}}}\,ArI}}\,\]
(3) Physical properties
(i) Physical state : Haloarenes are colourless liquid or crystalline solid.
(ii) Solubility : They are insoluble in water, but dissolve readily in organic solvents. Insolubility is due to inability to form hydrogen bonding in water. Para isomer is less soluble than ortho isomer.
(iii) Halo-arenes are heavier than water.
(iv) B.P. of halo-arenes follow the trend. Iodo arene > Bromo arene > Chloro arene.
(4) Chemical properties
Inert nature of chlorobenzene : Aryl halides are unreactive as compared to alkyl halides as the halogen atom in these compounds is firmly attached and cannot be replaced by nucleophiles. Such as \[O{{H}^{-}},NH_{2}^{-},C{{N}^{-}}\] etc.
(iii) Hunsdiecker reaction :
\[{{C}_{6}}{{H}_{5}}CO{{O}^{-}}A{{g}^{+}}\xrightarrow{B{{r}_{2}}}{{C}_{6}}{{H}_{5}}Br+C{{O}_{2}}+AgBr\]
(iv) From Aryl thalium compound :
\[ArH+Tl{{(OOCC{{F}_{3}})}_{3}}\xrightarrow[-C{{F}_{3}}C{{O}_{2}}H]{}\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\text{Aryl thallium trifluoroacetate}}{\mathop{ArTl{{(OOC{{F}_{3}})}_{2}}\underset{\Delta }{\mathop{\xrightarrow{KI}}}\,ArI}}\,\] \[\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\text{Aryl thallium trifluoroacetate}}{\mathop{ArTl{{(OOC{{F}_{3}})}_{2}}\underset{\Delta }{\mathop{\xrightarrow{KI}}}\,ArI}}\,\]
(3) Physical properties
(i) Physical state : Haloarenes are colourless liquid or crystalline solid.
(ii) Solubility : They are insoluble in water, but dissolve readily in organic solvents. Insolubility is due to inability to form hydrogen bonding in water. Para isomer is less soluble than ortho isomer.
(iii) Halo-arenes are heavier than water.
(iv) B.P. of halo-arenes follow the trend. Iodo arene > Bromo arene > Chloro arene.
(4) Chemical properties
Inert nature of chlorobenzene : Aryl halides are unreactive as compared to alkyl halides as the halogen atom in these compounds is firmly attached and cannot be replaced by nucleophiles. Such as \[O{{H}^{-}},NH_{2}^{-},C{{N}^{-}}\] etc.
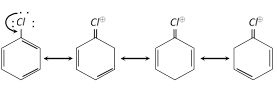 Thus delocalization of electrons by resonance in aryl halides, brings extra stability and double bond character between \[C-X\]bond. This makes the bond stronger and shorter than pure single bond. However under vigorous conditions the following nucleophilic substitution reactions are observed,
(i) Nucleophilic displacement :
\[{{C}_{6}}{{H}_{5}}Cl\underset{500\,atm.}{\mathop{\xrightarrow{NaOH,\,350{}^\circ C}}}\,{{C}_{6}}{{H}_{5}}OH+NaCl\]
(ii) Electrophilic aromatic substitution
Thus delocalization of electrons by resonance in aryl halides, brings extra stability and double bond character between \[C-X\]bond. This makes the bond stronger and shorter than pure single bond. However under vigorous conditions the following nucleophilic substitution reactions are observed,
(i) Nucleophilic displacement :
\[{{C}_{6}}{{H}_{5}}Cl\underset{500\,atm.}{\mathop{\xrightarrow{NaOH,\,350{}^\circ C}}}\,{{C}_{6}}{{H}_{5}}OH+NaCl\]
(ii) Electrophilic aromatic substitution
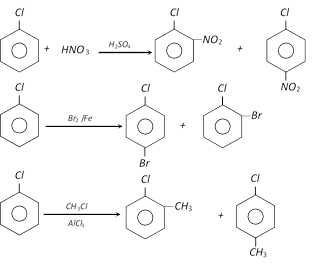 (iii) Wurtz – fittig reaction :
\[{{C}_{6}}{{H}_{5}}Br+C{{H}_{3}}Br\underset{\text{Ether}}{\mathop{\xrightarrow{Na}}}\,{{C}_{6}}{{H}_{5}}C{{H}_{3}}+2NaBr\]
(iv) Formation of grignard reagent :
\[{{C}_{6}}{{H}_{5}}Br\underset{\text{Ether}}{\mathop{\xrightarrow{Mg}}}\,{{C}_{6}}{{H}_{5}}MgBr\]
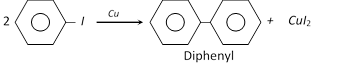
(v) Ullmann reaction
(iii) Wurtz – fittig reaction :
\[{{C}_{6}}{{H}_{5}}Br+C{{H}_{3}}Br\underset{\text{Ether}}{\mathop{\xrightarrow{Na}}}\,{{C}_{6}}{{H}_{5}}C{{H}_{3}}+2NaBr\]
(iv) Formation of grignard reagent :
\[{{C}_{6}}{{H}_{5}}Br\underset{\text{Ether}}{\mathop{\xrightarrow{Mg}}}\,{{C}_{6}}{{H}_{5}}MgBr\]
(v) Ullmann reaction
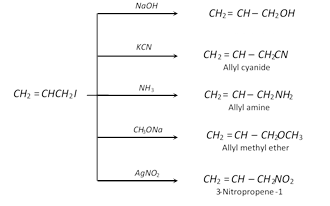 Addition reactions : Electrophilic addition reactions take place in accordance to Markownikoff's rule.
\[C{{H}_{2}}=CH-C{{H}_{2}}I+B{{r}_{2}}\xrightarrow{{}}\underset{\text{1,2}-\text{Dibromo-3-iodopropane}}{\mathop{C{{H}_{2}}Br\cdot CHBr\cdot C{{H}_{2}}I}}\,\]
\[C{{H}_{2}}=CH-C{{H}_{2}}I+HBr\xrightarrow{{}}\underset{\text{2}-\text{Bromo-1-iodopropane}}{\mathop{C{{H}_{3}}CHBrC{{H}_{2}}I}}\,\]
Allyl iodide is widely used in organic synthesis.
Addition reactions : Electrophilic addition reactions take place in accordance to Markownikoff's rule.
\[C{{H}_{2}}=CH-C{{H}_{2}}I+B{{r}_{2}}\xrightarrow{{}}\underset{\text{1,2}-\text{Dibromo-3-iodopropane}}{\mathop{C{{H}_{2}}Br\cdot CHBr\cdot C{{H}_{2}}I}}\,\]
\[C{{H}_{2}}=CH-C{{H}_{2}}I+HBr\xrightarrow{{}}\underset{\text{2}-\text{Bromo-1-iodopropane}}{\mathop{C{{H}_{3}}CHBrC{{H}_{2}}I}}\,\]
Allyl iodide is widely used in organic synthesis.
 more...
more... 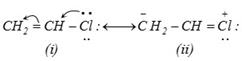 The following two effects are observed due to resonance stabilization.
(i) Carbon-chlorine bond in vinyl chloride has some double bond character and is, therefore, stronger than a pure single bond.
(ii) Carbon atom is \[s{{p}^{2}}\] hybridized and \[C-Cl\] bond length is shorter \[(1.69{\AA})\] and the bond is stronger than in alkyl halides (1.80Å) due to \[s{{p}^{3}}\] hybridization of the carbon atom.
Addition reactions
The following two effects are observed due to resonance stabilization.
(i) Carbon-chlorine bond in vinyl chloride has some double bond character and is, therefore, stronger than a pure single bond.
(ii) Carbon atom is \[s{{p}^{2}}\] hybridized and \[C-Cl\] bond length is shorter \[(1.69{\AA})\] and the bond is stronger than in alkyl halides (1.80Å) due to \[s{{p}^{3}}\] hybridization of the carbon atom.
Addition reactions
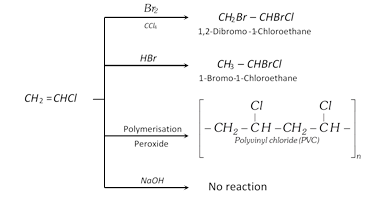 (3) Uses : The main use of vinyl chloride is in the manufacture of polyvinyl chloride (PVC) plastic which is employed these days for making synthetic leather goods, rain coats, pipes, floor tiles, gramophone records, packaging materials, etc.
(3) Uses : The main use of vinyl chloride is in the manufacture of polyvinyl chloride (PVC) plastic which is employed these days for making synthetic leather goods, rain coats, pipes, floor tiles, gramophone records, packaging materials, etc.
You need to login to perform this action.
You will be redirected in
3 sec
