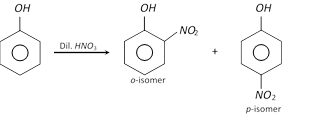
 (ii)
(ii) 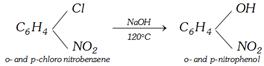 (iii)
(iii) 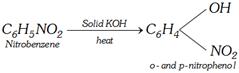 (iv)
(iv) 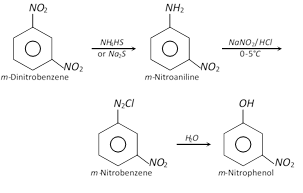 (2) Properties : o-Nitrophenol is a yellow coloured crystalline compound, while m- and p-isomers are colourless crystalline compounds.
\[\begin{matrix} \text{Isomer} \\ \text{m}\text{.pt}\text{. (}{}^\circ \text{C)} \\ \end{matrix}\,\,\,\,\,\,\begin{matrix} ortho \\ 45 \\ \end{matrix}\,\,\,\,\,\,\begin{matrix} meta \\ 97 \\ \end{matrix}\,\,\,\,\,\,\begin{matrix} para \\ 114 \\ \end{matrix}\]
The lowest melting point of o-isomer is due to intramolecular hydrogen bonding whereas meta and para isomers possess intermolecular hydrogen bonding and thus, they have higher melting points.
They are stronger acids than phenol. The order is :
p-isomer > o-isomer > m-isomer > phenol
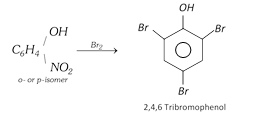
When reduced, they form corresponding aminophenols. o- and p-Nitrophenols react with bromine water to form 2, 4, 6-tribromophenol by replacement of nitro group.
(2) Properties : o-Nitrophenol is a yellow coloured crystalline compound, while m- and p-isomers are colourless crystalline compounds.
\[\begin{matrix} \text{Isomer} \\ \text{m}\text{.pt}\text{. (}{}^\circ \text{C)} \\ \end{matrix}\,\,\,\,\,\,\begin{matrix} ortho \\ 45 \\ \end{matrix}\,\,\,\,\,\,\begin{matrix} meta \\ 97 \\ \end{matrix}\,\,\,\,\,\,\begin{matrix} para \\ 114 \\ \end{matrix}\]
The lowest melting point of o-isomer is due to intramolecular hydrogen bonding whereas meta and para isomers possess intermolecular hydrogen bonding and thus, they have higher melting points.
They are stronger acids than phenol. The order is :
p-isomer > o-isomer > m-isomer > phenol
When reduced, they form corresponding aminophenols. o- and p-Nitrophenols react with bromine water to form 2, 4, 6-tribromophenol by replacement of nitro group.
 Picric acid (2, 4, 6-trinitrophenol)
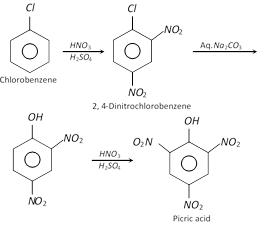
(1) Preparation : It is obtained when phenol is treated with conc. \[HN{{O}_{3}}\]. However, the yield is very poor. It is prepared on an industrial scale :
(i) From chlorobenzene
Picric acid (2, 4, 6-trinitrophenol)
(1) Preparation : It is obtained when phenol is treated with conc. \[HN{{O}_{3}}\]. However, the yield is very poor. It is prepared on an industrial scale :
(i) From chlorobenzene
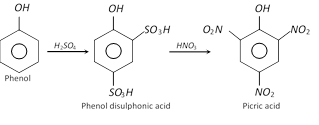
 (ii) From phenol through disulphonic acid
(ii) From phenol through disulphonic acid
 (iii)
(iii)
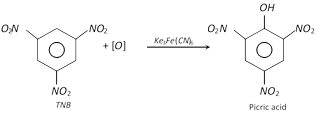 (2) Properties : It is a yellow crystalline solid, melting points \[{{122}^{o}}C\]. it is insoluble in cold water but soluble in hot water and in ether. It is bitter in taste. Due to the presence of three electronegative nitro groups, it is a stronger acid than phenol and its properties are comparable to the carboxylic acid. It neutralises alkalies and decomposes carbonates with evolution of carbon dioxide.
Dry picric acid as well as its potassium or ammonium salts explode violently when detonated. It reacts with \[PC{{l}_{5}}\] to form picryl chloride which on shaking with \[N{{H}_{3}}\] yields picramide.
(2) Properties : It is a yellow crystalline solid, melting points \[{{122}^{o}}C\]. it is insoluble in cold water but soluble in hot water and in ether. It is bitter in taste. Due to the presence of three electronegative nitro groups, it is a stronger acid than phenol and its properties are comparable to the carboxylic acid. It neutralises alkalies and decomposes carbonates with evolution of carbon dioxide.
Dry picric acid as well as its potassium or ammonium salts explode violently when detonated. It reacts with \[PC{{l}_{5}}\] to form picryl chloride which on shaking with \[N{{H}_{3}}\] yields picramide.
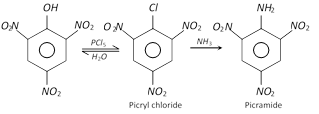 When distilled with a paste of bleaching powder, it gets decomposed and yields chloropicrin, \[CC{{l}_{3}}N{{O}_{2}}\], as one of the products and is thus employed for the manufacture of tear gas.
It forms yellow, orange or red coloured molecular compounds called picrates with aromatic hydrocarbons, amines and phenols which are used for characterisation of these compounds.
When distilled with a paste of bleaching powder, it gets decomposed and yields chloropicrin, \[CC{{l}_{3}}N{{O}_{2}}\], as one of the products and is thus employed for the manufacture of tear gas.
It forms yellow, orange or red coloured molecular compounds called picrates with aromatic hydrocarbons, amines and phenols which are used for characterisation of these compounds.
 (ii)
(ii)  (iii)
(iii)  (2) Properties : more...
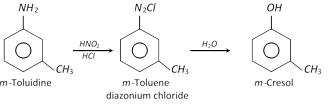
(2) Properties : more... 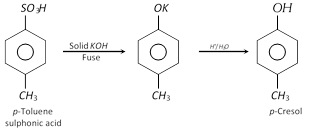 (ii) From benzene diazonium chloride
\[\underset{\text{Benzene}}{\mathop{{{C}_{6}}{{H}_{6}}}}\,\underset{{{H}_{2}}S{{O}_{4}},\,{{45}^{o}}C}{\mathop{\xrightarrow{HN{{O}_{3}}}}}\,\underset{\text{Nitrobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}N{{O}_{2}}}}\,\xrightarrow{Sn/HCl}\underset{\text{Aniline}}{\mathop{{{C}_{6}}{{H}_{5}}N{{H}_{2}}}}\,\]
\[\underset{HCl,\,0-{{5}^{o}}C}{\mathop{\xrightarrow{NaN{{O}_{2}}}}}\,\underset{\text{Benzene}\,\text{diazonium}\,\text{chloride}}{\mathop{{{C}_{6}}{{H}_{5}}{{N}_{2}}Cl}}\,\underset{\text{Warm}}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\underset{\text{Phenol}}{\mathop{{{C}_{6}}{{H}_{5}}OH}}\,\]
(ii) From benzene diazonium chloride
\[\underset{\text{Benzene}}{\mathop{{{C}_{6}}{{H}_{6}}}}\,\underset{{{H}_{2}}S{{O}_{4}},\,{{45}^{o}}C}{\mathop{\xrightarrow{HN{{O}_{3}}}}}\,\underset{\text{Nitrobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}N{{O}_{2}}}}\,\xrightarrow{Sn/HCl}\underset{\text{Aniline}}{\mathop{{{C}_{6}}{{H}_{5}}N{{H}_{2}}}}\,\]
\[\underset{HCl,\,0-{{5}^{o}}C}{\mathop{\xrightarrow{NaN{{O}_{2}}}}}\,\underset{\text{Benzene}\,\text{diazonium}\,\text{chloride}}{\mathop{{{C}_{6}}{{H}_{5}}{{N}_{2}}Cl}}\,\underset{\text{Warm}}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\underset{\text{Phenol}}{\mathop{{{C}_{6}}{{H}_{5}}OH}}\,\]
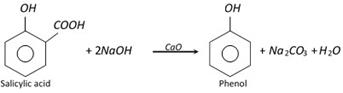 (v) Middle oil of coal tar distillation : Middle oil of coal-tar distillation has naphthalene and phenolic compounds. Phenolic compounds are isolated in following steps.
Step I : Middle oil is washed with \[{{H}_{2}}S{{O}_{4}}\]. It dissolves basic impurities like pyridine (base).
Step II : Ecessive cooling separates naphthalene (a low melting solid)
Step III : Filtrate of step II is treated with aqueous NaOH when phenols dissolve as phenoxides. Carbon dioxide is then blown through the solution to liberate phenols.
\[{{C}_{6}}{{H}_{5}}OH+NaOH\to {{C}_{6}}{{H}_{5}}ONa+{{H}_{2}}O\]\[\xrightarrow{C{{O}_{2}},\,{{H}_{2}}O}{{C}_{6}}{{H}_{5}}OH+N{{a}_{2}}C{{O}_{3}}\]
Step IV : Crude phenol (of step III) is subjected to fractional distillation.
(v) Middle oil of coal tar distillation : Middle oil of coal-tar distillation has naphthalene and phenolic compounds. Phenolic compounds are isolated in following steps.
Step I : Middle oil is washed with \[{{H}_{2}}S{{O}_{4}}\]. It dissolves basic impurities like pyridine (base).
Step II : Ecessive cooling separates naphthalene (a low melting solid)
Step III : Filtrate of step II is treated with aqueous NaOH when phenols dissolve as phenoxides. Carbon dioxide is then blown through the solution to liberate phenols.
\[{{C}_{6}}{{H}_{5}}OH+NaOH\to {{C}_{6}}{{H}_{5}}ONa+{{H}_{2}}O\]\[\xrightarrow{C{{O}_{2}},\,{{H}_{2}}O}{{C}_{6}}{{H}_{5}}OH+N{{a}_{2}}C{{O}_{3}}\]
Step IV : Crude phenol (of step III) is subjected to fractional distillation.
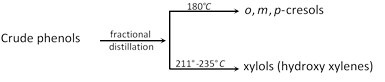 (vi) Raschig’s process
\[\underset{\text{Benzene}}{\mathop{{{C}_{6}}{{H}_{6}}}}\,+HCl+\frac{1}{2}{{O}_{2}}\underset{{{250}^{o}}C}{\mathop{\xrightarrow{CuC{{l}_{2}}/FeC{{l}_{3}}}}}\,\underset{\text{Chlorobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}Cl}}\,+{{H}_{2}}O\]
\[\underset{\text{Chlorobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}Cl}}\,+\underset{\text{steam}}{\mathop{{{H}_{2}}O}}\,\xrightarrow{{{425}^{o}}C}\underset{\text{Phenol}}{\mathop{{{C}_{6}}{{H}_{5}}OH}}\,\,\,+HCl\]
(vii) Dow process
\[\underset{\text{Chlorobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}Cl}}\,+2NaOH\underset{\text{High pressure}}{\mathop{\xrightarrow{{{300}^{o}}C}}}\,{{C}_{6}}{{H}_{5}}ONa+NaCl+{{H}_{2}}O\]
sodium phenoxide on treatment with mineral acid yields phenol.
\[2{{C}_{6}}{{H}_{5}}ONa+{{H}_{2}}S{{O}_{4}}\to 2{{C}_{6}}{{H}_{5}}OH+N{{a}_{2}}S{{O}_{4}}\]
(viii) Oxidation of benzene
\[2{{C}_{6}}{{H}_{6}}+{{O}_{2}}\underset{{{315}^{o}}C}{\mathop{\xrightarrow{{{V}_{2}}{{O}_{5}}}}}\,2{{C}_{6}}{{H}_{5}}OH\]
(ix) Oxidation of isopropyl benzene [Cumene]
(vi) Raschig’s process
\[\underset{\text{Benzene}}{\mathop{{{C}_{6}}{{H}_{6}}}}\,+HCl+\frac{1}{2}{{O}_{2}}\underset{{{250}^{o}}C}{\mathop{\xrightarrow{CuC{{l}_{2}}/FeC{{l}_{3}}}}}\,\underset{\text{Chlorobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}Cl}}\,+{{H}_{2}}O\]
\[\underset{\text{Chlorobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}Cl}}\,+\underset{\text{steam}}{\mathop{{{H}_{2}}O}}\,\xrightarrow{{{425}^{o}}C}\underset{\text{Phenol}}{\mathop{{{C}_{6}}{{H}_{5}}OH}}\,\,\,+HCl\]
(vii) Dow process
\[\underset{\text{Chlorobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}Cl}}\,+2NaOH\underset{\text{High pressure}}{\mathop{\xrightarrow{{{300}^{o}}C}}}\,{{C}_{6}}{{H}_{5}}ONa+NaCl+{{H}_{2}}O\]
sodium phenoxide on treatment with mineral acid yields phenol.
\[2{{C}_{6}}{{H}_{5}}ONa+{{H}_{2}}S{{O}_{4}}\to 2{{C}_{6}}{{H}_{5}}OH+N{{a}_{2}}S{{O}_{4}}\]
(viii) Oxidation of benzene
\[2{{C}_{6}}{{H}_{6}}+{{O}_{2}}\underset{{{315}^{o}}C}{\mathop{\xrightarrow{{{V}_{2}}{{O}_{5}}}}}\,2{{C}_{6}}{{H}_{5}}OH\]
(ix) Oxidation of isopropyl benzene [Cumene]
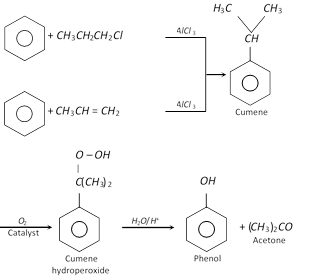 (2) Physical properties
(i) Phenol is a colourless crystalline, deliquescent solid. It attains pink colour on exposure to air and light.
(ii) They are capable of forming intermolecular H-bonding among themselves and with water. Thus, they have high boiling points and they are soluble in water.
(2) Physical properties
(i) Phenol is a colourless crystalline, deliquescent solid. It attains pink colour on exposure to air and light.
(ii) They are capable of forming intermolecular H-bonding among themselves and with water. Thus, they have high boiling points and they are soluble in water.
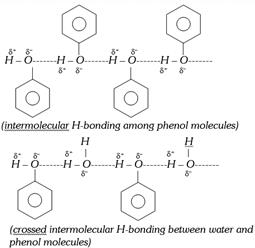 Due to intermolecular H-bonding and high dipole moment, melting points and boiling points of phenol are much higher than that of hydrocarbon of comparable molecular weights.
(iii) It has a peculiar characteristic smell and a strong corrosive action on skin.
(iv) It is sparingly soluble in water but readily soluble in organic solvents such as alcohol, benzene and ether.
(v) It is poisonous in nature but acts as antiseptic and disinfectant.
(3) Chemical properties
(i) Acidic nature : Phenol is a weak acid. The acidic nature of phenol is due to the formation of stable phenoxide ion in solution.
\[{{C}_{6}}{{H}_{5}}OH+{{H}_{2}}O\] ? \[\underset{\text{Phenoxide ion}}{\mathop{{{C}_{6}}{{H}_{5}}{{O}^{-}}}}\,+{{H}_{3}}{{O}^{+}}\]
The phenoxide ion is stable due to resonance.
Due to intermolecular H-bonding and high dipole moment, melting points and boiling points of phenol are much higher than that of hydrocarbon of comparable molecular weights.
(iii) It has a peculiar characteristic smell and a strong corrosive action on skin.
(iv) It is sparingly soluble in water but readily soluble in organic solvents such as alcohol, benzene and ether.
(v) It is poisonous in nature but acts as antiseptic and disinfectant.
(3) Chemical properties
(i) Acidic nature : Phenol is a weak acid. The acidic nature of phenol is due to the formation of stable phenoxide ion in solution.
\[{{C}_{6}}{{H}_{5}}OH+{{H}_{2}}O\] ? \[\underset{\text{Phenoxide ion}}{\mathop{{{C}_{6}}{{H}_{5}}{{O}^{-}}}}\,+{{H}_{3}}{{O}^{+}}\]
The phenoxide ion is stable due to resonance.
 (viii) Reaction with nitric acid
\[\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OH\,}}}\,\,\,+\,\,3HN{{O}_{3}}\xrightarrow{\text{conc}\text{. }{{H}_{2}}S{{O}_{4}}}\underset{\text{Glyceryl trinitrate (T}\text{.N}\text{.G}\text{.)}}{\mathop{\underset{C{{H}_{2}}ON{{O}_{2}}}{\overset{C{{H}_{2}}ON{{O}_{2}}}{\mathop{\underset{|\,\,\,\,\,\,\,}{\overset{|\,\,\,\,\,\,\,}{\mathop{CH}}}\,ON{{O}_{2}}}}}\,\,\,+\,\,3{{H}_{2}}O}}\,\]
Dynamite is prepared from T.N.G.
Dynamite : A mixture of T.N.G. and glyceryl dinitrate absorbed in kieselguhr is called dynamite. It was discovered by Alfred. Nobel in 1867. It releases large volume of gases and occupy 10,900 times the volume of nitroglycerine.
\[{{C}_{3}}{{H}_{5}}{{(ONO)}_{3}}\to 12C{{O}_{2}}+10{{H}_{2}}O+6{{N}_{2}}+{{O}_{2}}\]
Blasting gelatin : A mixture of glyceryl trinitrate and cellulose nitrate (gun cotton).
Cordite : It is obtained by mixing glyceryl trinitrate with gun cotton and vaseline it is smokeless explosive.
(4) Uses
(a) As antifreeze in automobile radiator.
(b) In the preparation of good quality of soap-hand lotions shaving creams and tooth pastes.
(c) As a lubricant in watches.
(d) As a preservatives.
(e) As a sweetening agent in confectionary, beverages and medicines being non toxic in nature.
(f) In manufacture of explosives such as dynamite.
(5) Analytical tests of glycerol
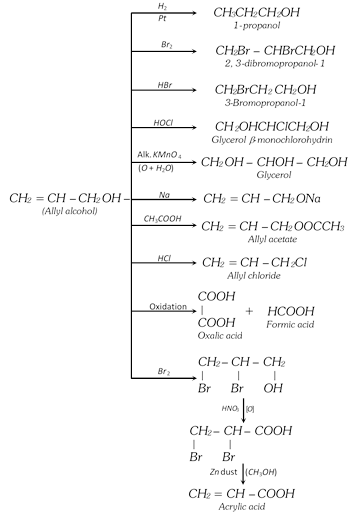
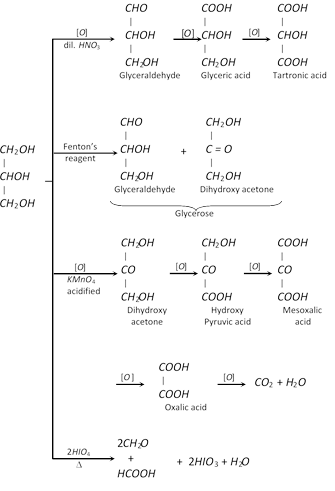
(i) Acrolein test : When glycerol is heated with \[KHS{{O}_{4}}\] a very offensive smell is produced due to formation of acrolein. Its aqueous solution restores the colour of schiff’s reagent and reduces Fehling solution and Tollen’s reagent.
(ii) Dunstan’s test : A drop of phenolphthalein is added approximately 5 ml of borax solution. The pink colour appears on adding 2-3 more...
(viii) Reaction with nitric acid
\[\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{CH}}}\,OH\,}}}\,\,\,+\,\,3HN{{O}_{3}}\xrightarrow{\text{conc}\text{. }{{H}_{2}}S{{O}_{4}}}\underset{\text{Glyceryl trinitrate (T}\text{.N}\text{.G}\text{.)}}{\mathop{\underset{C{{H}_{2}}ON{{O}_{2}}}{\overset{C{{H}_{2}}ON{{O}_{2}}}{\mathop{\underset{|\,\,\,\,\,\,\,}{\overset{|\,\,\,\,\,\,\,}{\mathop{CH}}}\,ON{{O}_{2}}}}}\,\,\,+\,\,3{{H}_{2}}O}}\,\]
Dynamite is prepared from T.N.G.
Dynamite : A mixture of T.N.G. and glyceryl dinitrate absorbed in kieselguhr is called dynamite. It was discovered by Alfred. Nobel in 1867. It releases large volume of gases and occupy 10,900 times the volume of nitroglycerine.
\[{{C}_{3}}{{H}_{5}}{{(ONO)}_{3}}\to 12C{{O}_{2}}+10{{H}_{2}}O+6{{N}_{2}}+{{O}_{2}}\]
Blasting gelatin : A mixture of glyceryl trinitrate and cellulose nitrate (gun cotton).
Cordite : It is obtained by mixing glyceryl trinitrate with gun cotton and vaseline it is smokeless explosive.
(4) Uses
(a) As antifreeze in automobile radiator.
(b) In the preparation of good quality of soap-hand lotions shaving creams and tooth pastes.
(c) As a lubricant in watches.
(d) As a preservatives.
(e) As a sweetening agent in confectionary, beverages and medicines being non toxic in nature.
(f) In manufacture of explosives such as dynamite.
(5) Analytical tests of glycerol
(i) Acrolein test : When glycerol is heated with \[KHS{{O}_{4}}\] a very offensive smell is produced due to formation of acrolein. Its aqueous solution restores the colour of schiff’s reagent and reduces Fehling solution and Tollen’s reagent.
(ii) Dunstan’s test : A drop of phenolphthalein is added approximately 5 ml of borax solution. The pink colour appears on adding 2-3 more... 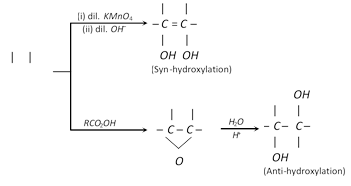 (b) With \[{{O}_{2}}\] in presence of Ag :
(b) With \[{{O}_{2}}\] in presence of Ag :
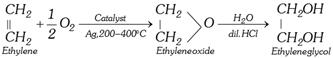 (c) With HOCl followed by hydrolysis : (Industrial method)
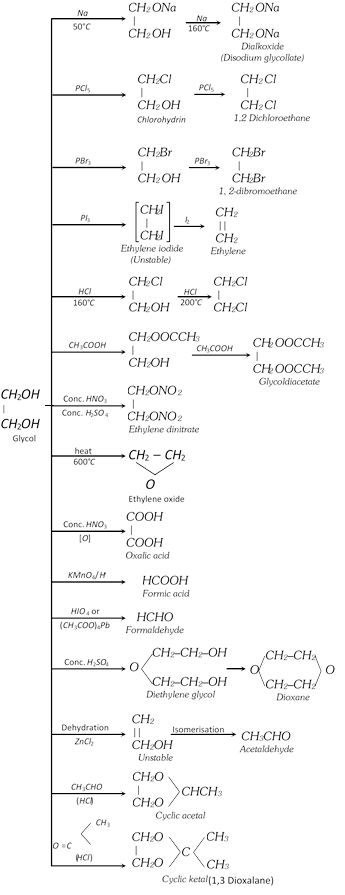
\[\begin{matrix}\underset{|\,|\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\, \\C{{H}_{2}} \\\end{matrix}+HOCl\to \underset{\text{Ethylenechlorohydrin}}{\mathop{\begin{matrix}\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}OH}}\, \\C{{H}_{2}}Cl\,\,\, \\\end{matrix}}}\,\]\[\xrightarrow{NaHC{{O}_{3}}}\underset{\text{Glycol}}{\mathop{\begin{matrix}\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}OH}}\, \\C{{H}_{2}}OH \\\end{matrix}}}\,+NaCl+C{{O}_{2}}\]
(ii) From 1, 2 dibromo ethane [Lab method]:
\[\begin{matrix}\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}Br}}\,\\C{{H}_{2}}Br\\\end{matrix}+N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O\to\begin{matrix}\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}OH}}\,\\C{{H}_{2}}OH\\\end{matrix}+2NaBr+C{{O}_{2}}\]
\[\begin{matrix}\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}Br}}\,\\C{{H}_{2}}Br\\\end{matrix}+2C{{H}_{3}}COOK\underset{-2KBr}{\mathop{\xrightarrow{C{{H}_{3}}COOH}}}\,\underset{\text{Glycoldiacetate}}{\mathop{\begin{matrix}\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}OOCC{{H}_{3}}}}\,\\C{{H}_{2}}OOCC{{H}_{3}}\\\end{matrix}}}\,\xrightarrow{NaOH}\begin{matrix}\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}OH}}\,\\C{{H}_{2}}OH\\\end{matrix}+2C{{H}_{3}}COONa\]
(2) Physical properties
(i) It is a colourless, syrupy liquid and sweet in taste. Its boiling point is \[{{197}^{o}}C\].
(ii) It is miscible in water and ethanol in all proportions but is insoluble in ether.
(iii) It is toxic as methanol when taken orally.
(iv) It is widely used as a solvent and as an antifreeze agent.
(3) Chemical properties
(c) With HOCl followed by hydrolysis : (Industrial method)
\[\begin{matrix}\underset{|\,|\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\, \\C{{H}_{2}} \\\end{matrix}+HOCl\to \underset{\text{Ethylenechlorohydrin}}{\mathop{\begin{matrix}\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}OH}}\, \\C{{H}_{2}}Cl\,\,\, \\\end{matrix}}}\,\]\[\xrightarrow{NaHC{{O}_{3}}}\underset{\text{Glycol}}{\mathop{\begin{matrix}\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}OH}}\, \\C{{H}_{2}}OH \\\end{matrix}}}\,+NaCl+C{{O}_{2}}\]
(ii) From 1, 2 dibromo ethane [Lab method]:
\[\begin{matrix}\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}Br}}\,\\C{{H}_{2}}Br\\\end{matrix}+N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O\to\begin{matrix}\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}OH}}\,\\C{{H}_{2}}OH\\\end{matrix}+2NaBr+C{{O}_{2}}\]
\[\begin{matrix}\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}Br}}\,\\C{{H}_{2}}Br\\\end{matrix}+2C{{H}_{3}}COOK\underset{-2KBr}{\mathop{\xrightarrow{C{{H}_{3}}COOH}}}\,\underset{\text{Glycoldiacetate}}{\mathop{\begin{matrix}\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}OOCC{{H}_{3}}}}\,\\C{{H}_{2}}OOCC{{H}_{3}}\\\end{matrix}}}\,\xrightarrow{NaOH}\begin{matrix}\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}OH}}\,\\C{{H}_{2}}OH\\\end{matrix}+2C{{H}_{3}}COONa\]
(2) Physical properties
(i) It is a colourless, syrupy liquid and sweet in taste. Its boiling point is \[{{197}^{o}}C\].
(ii) It is miscible in water and ethanol in all proportions but is insoluble in ether.
(iii) It is toxic as methanol when taken orally.
(iv) It is widely used as a solvent and as an antifreeze agent.
(3) Chemical properties
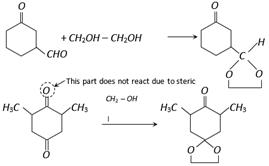
 Dioxalane formation provides a path of protecting a carbonyl group in reaction studied in basic medium in which acetals are not affected. The carbonyl compound may be regenerated by the addition of periodic acid to aqueous solution of the dioxalane or by acidic hydrolysis.
Dioxalane formation provides a path of protecting a carbonyl group in reaction studied in basic medium in which acetals are not affected. The carbonyl compound may be regenerated by the addition of periodic acid to aqueous solution of the dioxalane or by acidic hydrolysis.
 (4) Uses
(i) Used as an antifreeze in car radiators.
(ii) Used in the manufacture of dacron, dioxane etc.
(iii) As a solvent and as a preservatives.
(iv) As a cooling agent in aeroplanes.
(v) As an explosives in the form of dinitrate.
(4) Uses
(i) Used as an antifreeze in car radiators.
(ii) Used in the manufacture of dacron, dioxane etc.
(iii) As a solvent and as a preservatives.
(iv) As a cooling agent in aeroplanes.
(v) As an explosives in the form of dinitrate.
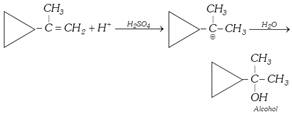 (b) Oxymercuration-demercuration
(b) Oxymercuration-demercuration
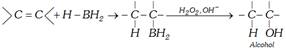 Diborane is an electron defficient molecule. It acts as an electrophile reacting with alkenes to form alkyl boranes \[{{R}_{3}}B\].
\[R-CH=C{{H}_{2}}+H-B{{H}_{2}}\to \underset{\text{Alkyl borane}}{\mathop{R-\underset{H\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,}{\mathop{CH}}\,}}\,-\underset{B\,{{H}_{2}}\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,}}\,\xrightarrow{RCH=C{{H}_{2}}}\]
\[\underset{\text{Dialkyl borane}}{\mathop{{{(R\,C{{H}_{2}}\,C{{H}_{2}})}_{2}}}}\,BH\xrightarrow{RCH=C{{H}_{2}}}\underset{\text{Trialkyl borane}}{\mathop{{{(RC{{H}_{2}}C{{H}_{2}})}_{3}}B}}\,\]
Diborane is an electron defficient molecule. It acts as an electrophile reacting with alkenes to form alkyl boranes \[{{R}_{3}}B\].
\[R-CH=C{{H}_{2}}+H-B{{H}_{2}}\to \underset{\text{Alkyl borane}}{\mathop{R-\underset{H\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,}{\mathop{CH}}\,}}\,-\underset{B\,{{H}_{2}}\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,}}\,\xrightarrow{RCH=C{{H}_{2}}}\]
\[\underset{\text{Dialkyl borane}}{\mathop{{{(R\,C{{H}_{2}}\,C{{H}_{2}})}_{2}}}}\,BH\xrightarrow{RCH=C{{H}_{2}}}\underset{\text{Trialkyl borane}}{\mathop{{{(RC{{H}_{2}}C{{H}_{2}})}_{3}}B}}\,\]
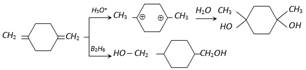 \[LiAl{{H}_{4}}\] also reduces epoxides into alcohol :
\[LiAl{{H}_{4}}\] also reduces epoxides into alcohol :
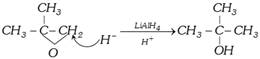 (iv) By reduction of carboxylic acids and their derivatives
\[\underset{\text{Carboxylic acid}}{\mathop{R-COOH}}\,\underset{\text{(ii) }{{H}_{2}}O}{\mathop{\xrightarrow{\text{(i) }LiAl{{H}_{4}}}}}\,\underset{\text{primary alcohol}}{\mathop{RC{{H}_{2}}OH}}\,\]
\[\underset{\text{Carboxylic acid}}{\mathop{RCOOH}}\,\to \underset{\text{Ester}}{\mathop{RCOO{R}'}}\,\underset{\text{Catalyst}}{\mathop{\xrightarrow{{{H}_{2}}}}}\,RC{{H}_{2}}OH+{R}'OH\]
Esters are also reduced to alcohols
(Bouveault Blanc reaction)
\[\underset{\begin{smallmatrix}\text{Methyl acetate} \\\text{ (Ester)}\end{smallmatrix}}{\mathop{C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-OC{{H}_{3}}}}\,\,+4[H]\xrightarrow{Na/{{C}_{2}}{{H}_{5}}OH}\underset{\text{Ethanol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\,+\,\underset{\text{Methanol}}{\mathop{C{{H}_{3}}OH}}\,\]
(iv) By reduction of carboxylic acids and their derivatives
\[\underset{\text{Carboxylic acid}}{\mathop{R-COOH}}\,\underset{\text{(ii) }{{H}_{2}}O}{\mathop{\xrightarrow{\text{(i) }LiAl{{H}_{4}}}}}\,\underset{\text{primary alcohol}}{\mathop{RC{{H}_{2}}OH}}\,\]
\[\underset{\text{Carboxylic acid}}{\mathop{RCOOH}}\,\to \underset{\text{Ester}}{\mathop{RCOO{R}'}}\,\underset{\text{Catalyst}}{\mathop{\xrightarrow{{{H}_{2}}}}}\,RC{{H}_{2}}OH+{R}'OH\]
Esters are also reduced to alcohols
(Bouveault Blanc reaction)
\[\underset{\begin{smallmatrix}\text{Methyl acetate} \\\text{ (Ester)}\end{smallmatrix}}{\mathop{C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-OC{{H}_{3}}}}\,\,+4[H]\xrightarrow{Na/{{C}_{2}}{{H}_{5}}OH}\underset{\text{Ethanol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\,+\,\underset{\text{Methanol}}{\mathop{C{{H}_{3}}OH}}\,\]
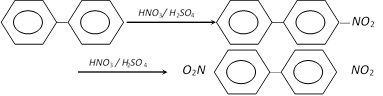
 (ii) Decarboxylation of cinnamic acid : This is the laboratory preparation and involves heating of cinnamic acid with a small amount of quinol.
\[{{C}_{6}}{{H}_{5}}CH=CHCOOH\xrightarrow{\text{Quinol}}{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}+C{{O}_{2}}\]
(iii) Dehydration of 1-phenyl ethanol with \[{{H}_{2}}S{{O}_{4}}\] :
\[{{C}_{6}}{{H}_{5}}CHOHC{{H}_{3}}\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}}}}\,{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\]
(iv) Dehydration of 2-phenyl ethanol with \[ZnC{{l}_{2}}\] :
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}C{{H}_{2}}OH\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{ZnC{{l}_{2}},\,\text{heat}}}}\,{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\]
(v) Dehydrohalogenation of 1-phenyl-1-chloro ethane : On heating with alcoholic potassium hydroxide, a molecule of hydrogen chloride is eliminated by the chloroderivative.
\[{{C}_{6}}{{H}_{5}}CHClC{{H}_{3}}\underset{\text{Heat}}{\mathop{\xrightarrow{\text{Alc}\text{.}\,KOH}}}\,{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\]
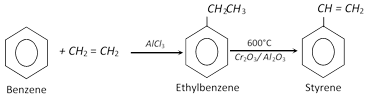
(2) Properties : It is a colourless liquid, boiling point \[{{145}^{o}}C\]. On keeping, it gradually changes into a solid polymer called metastyrene. The polymerisation is rapid in sunlight or when treated with sodium. It shows properties of benzene ring (Electrophilic substitution) and unsaturated side chain (Electrophilic addition). However, the side chain double bond is more susceptible to electrophilic attack as compared to benzene ring.
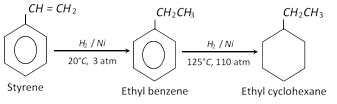
At lower temperature and pressure, it reacts with hydrogen to produce ethylbenzene and at higher temperature and pressure, it is converted into ethyl cyclohexane.
(ii) Decarboxylation of cinnamic acid : This is the laboratory preparation and involves heating of cinnamic acid with a small amount of quinol.
\[{{C}_{6}}{{H}_{5}}CH=CHCOOH\xrightarrow{\text{Quinol}}{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}+C{{O}_{2}}\]
(iii) Dehydration of 1-phenyl ethanol with \[{{H}_{2}}S{{O}_{4}}\] :
\[{{C}_{6}}{{H}_{5}}CHOHC{{H}_{3}}\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}}}}\,{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\]
(iv) Dehydration of 2-phenyl ethanol with \[ZnC{{l}_{2}}\] :
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}C{{H}_{2}}OH\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{ZnC{{l}_{2}},\,\text{heat}}}}\,{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\]
(v) Dehydrohalogenation of 1-phenyl-1-chloro ethane : On heating with alcoholic potassium hydroxide, a molecule of hydrogen chloride is eliminated by the chloroderivative.
\[{{C}_{6}}{{H}_{5}}CHClC{{H}_{3}}\underset{\text{Heat}}{\mathop{\xrightarrow{\text{Alc}\text{.}\,KOH}}}\,{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\]
(2) Properties : It is a colourless liquid, boiling point \[{{145}^{o}}C\]. On keeping, it gradually changes into a solid polymer called metastyrene. The polymerisation is rapid in sunlight or when treated with sodium. It shows properties of benzene ring (Electrophilic substitution) and unsaturated side chain (Electrophilic addition). However, the side chain double bond is more susceptible to electrophilic attack as compared to benzene ring.
At lower temperature and pressure, it reacts with hydrogen to produce ethylbenzene and at higher temperature and pressure, it is converted into ethyl cyclohexane.
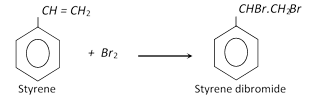
 With bromine, it gives the dibromide.
With bromine, it gives the dibromide.
 Halogen acids add to the side chain.
\[{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}+HX\xrightarrow{{}}{{C}_{6}}{{H}_{5}}CHXC{{H}_{3}}\]
Preparation of ring substituted styrenes is not done by direct halogenation but through indirect route.
Halogen acids add to the side chain.
\[{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}+HX\xrightarrow{{}}{{C}_{6}}{{H}_{5}}CHXC{{H}_{3}}\]
Preparation of ring substituted styrenes is not done by direct halogenation but through indirect route.
 When oxidised under drastic conditions, the side chain is completely oxidised to a carboxyl group.
When oxidised under drastic conditions, the side chain is completely oxidised to a carboxyl group.
 In presence of peroxides, styrene undergoes free radical polymerisation resulting in the formation of polystyrene ? an industrially important plastic.
\[n{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\xrightarrow{\text{Peroxide}}{{\left[ \underset{{{C}_{6}}{{H}_{5\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{-CH-C{{H}_{2}}-}}\,}}\, \right]}_{n}}\]
Co-polymers of styrene with butadiene and other substances are also important since many of them are industrially useful products such as SBR (A rubber substitute).
In presence of peroxides, styrene undergoes free radical polymerisation resulting in the formation of polystyrene ? an industrially important plastic.
\[n{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\xrightarrow{\text{Peroxide}}{{\left[ \underset{{{C}_{6}}{{H}_{5\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{-CH-C{{H}_{2}}-}}\,}}\, \right]}_{n}}\]
Co-polymers of styrene with butadiene and other substances are also important since many of them are industrially useful products such as SBR (A rubber substitute).
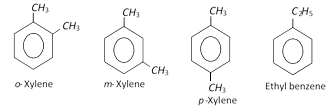 These are produced along with benzene, toluene and ethylbenzene when aromatisation of \[{{C}_{6}}-{{C}_{8}}\] fraction of petroleum naphtha is done. The xylenes are isolated from the resulting mixture (BTX) by fractional distillation.
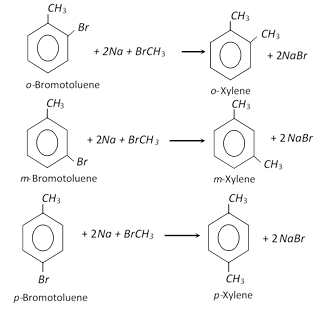
These can be prepared by Wurtz ? Fittig reaction. A mixture of bromotoluene and methylbromide is treated with sodium in dry ethereal solution to form the desired xylene.
These are produced along with benzene, toluene and ethylbenzene when aromatisation of \[{{C}_{6}}-{{C}_{8}}\] fraction of petroleum naphtha is done. The xylenes are isolated from the resulting mixture (BTX) by fractional distillation.
These can be prepared by Wurtz ? Fittig reaction. A mixture of bromotoluene and methylbromide is treated with sodium in dry ethereal solution to form the desired xylene.
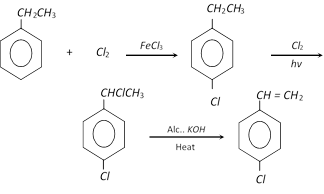
 These can also be obtained by Friedel – craft's synthesis,
m-Xylene can be obtained from mesitylene.
Xylenes are colourless liquids having characteristic odour. The boiling points of three isomers are,
o-Xylene=144°C; m-Xylene=139°C; p-Xylene=138°C.
Xylenes undergo electrophilic substitution reactions in the same manner as toluene. Upon oxidation with \[KMn{{O}_{4}}\] or \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\], Xylenes form corresponding dicarboxylic acids.
These can also be obtained by Friedel – craft's synthesis,
m-Xylene can be obtained from mesitylene.
Xylenes are colourless liquids having characteristic odour. The boiling points of three isomers are,
o-Xylene=144°C; m-Xylene=139°C; p-Xylene=138°C.
Xylenes undergo electrophilic substitution reactions in the same manner as toluene. Upon oxidation with \[KMn{{O}_{4}}\] or \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\], Xylenes form corresponding dicarboxylic acids.
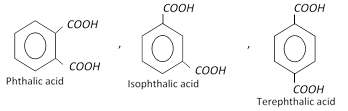 Xylenes are used in the manufacture of lacquers and as solvent for rubber. o-Xylene is used for the manufacture of phthalic anhydride.
Xylenes are used in the manufacture of lacquers and as solvent for rubber. o-Xylene is used for the manufacture of phthalic anhydride. You need to login to perform this action.
You will be redirected in
3 sec
