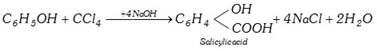 (4) Uses
(i) It is used as a fire extinguisher under the name pyrene. The dense vapours form a protective layer on the burning objects and prevent the oxygen or air to come in contact with the burning objects.
(ii) It is used as a solvent for fats, oils, waxes and greases, resins, iodine etc.
(iii) It finds use in medicine as helmenthicide for elimination of hook worms.
(4) Uses
(i) It is used as a fire extinguisher under the name pyrene. The dense vapours form a protective layer on the burning objects and prevent the oxygen or air to come in contact with the burning objects.
(ii) It is used as a solvent for fats, oils, waxes and greases, resins, iodine etc.
(iii) It finds use in medicine as helmenthicide for elimination of hook worms. | Colour | Salt | ||||||||||||||
| Black | Oxides : \[Mn{{O}_{2}},FeO,CuO,C{{o}_{3}}{{O}_{4}}\], \[N{{i}_{2}}{{O}_{3}}\] Sulphides : \[A{{g}_{2}}S,CuS,C{{u}_{2}}S,FeS,CoS,NiS\], \[PbS,HgS\],\[B{{i}_{2}}{{S}_{3}}\] (blackish brown) | ||||||||||||||
| Blue | Hydrated \[CuS{{O}_{4}}\], anhydrous \[CoS{{O}_{4}}\] | ||||||||||||||
| Orange | \[K{{O}_{2}}\], some dichromate \[({{K}_{2}}C{{r}_{2}}{{O}_{7}}),S{{b}_{2}}{{S}_{3}}\], ferricyanides | ||||||||||||||
| Green | Nickel salts, hydrated ferrous salts, potassium permanganate \[(KMn{{O}_{4}})\], some copper (II) salts | ||||||||||||||
| Brownish yellow | \[SnS\] | ||||||||||||||
| Dark brown | \[Pb{{O}_{2}},A{{g}_{2}}O,CdO,F{{e}_{2}}{{O}_{3}},CuCr{{O}_{4}},FeC{{l}_{3}}\] (but yellow in aq. solution) | ||||||||||||||
| Pale brown | \[MnC{{O}_{3}}\] | ||||||||||||||
|
Light pink
more...
(1) Colourless gases
(i) Tests for \[\mathbf{C}{{\mathbf{O}}_{\mathbf{2}}}\] : It is colourless and odourless gas. It gives white ppt. with lime water which dissolves on passing excess of \[C{{O}_{2}}\]. \[\underset{Lime\,water}{\mathop{Ca{{(OH)}_{2}}}}\,+C{{O}_{2}}\to \underset{White\,ppt.}{\mathop{CaC{{O}_{3}}}}\,\downarrow +{{H}_{2}}O\]
\[\underset{White\,ppt.}{\mathop{CaC{{O}_{3}}}}\,+\underset{Excess}{\mathop{C{{O}_{2}}}}\,+{{H}_{2}}O\to \underset{So\operatorname{lub}le}{\overset{{}}{\mathop{Ca{{(HC{{O}_{3}})}_{2}}}}}\,\]
(ii) Test for CO : It is colourless and odourless gas. It burns with a blue flame. \[2CO+{{O}_{2}}\to 2C{{O}_{2}}\]
(iii) Test for \[{{\mathbf{O}}_{\mathbf{2}}}\] : It is colourless and odourless gas. It rekindles a glowing splinter.
(iv) Tests for \[{{\mathbf{H}}_{\mathbf{2}}}\mathbf{S}\] : It is a colourless gas with a smell of rotten eggs. It turns moist lead acetate paper black.
\[{{(C{{H}_{3}}COO)}_{2}}Pb+{{H}_{2}}S\to 2C{{H}_{3}}COOH+\underset{Black}{\mathop{PbS}}\,\]
(v) Tests for \[\mathbf{S}{{\mathbf{O}}_{\mathbf{2}}}\] : It is a colourless gas with a suffocating odour of burning sulphur. It turns acidified \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\] solution green. \[3S{{O}_{2}}+{{K}_{2}}C{{r}_{2}}{{O}_{7}}+{{H}_{2}}S{{O}_{4}}\to {{K}_{2}}S{{O}_{4}}+\underset{Green}{\mathop{C{{r}_{2}}{{(S{{O}_{4}})}_{3}}}}\,+{{H}_{2}}O\]
(vi) Tests for \[\mathbf{N}{{\mathbf{H}}_{\mathbf{3}}}\]: It is a colourless gas with a characteristic ammonical smell. It gives white fumes of \[N{{H}_{4}}Cl\] with \[HCl\], \[N{{H}_{3}}+HCl\to \underset{White\,fumes}{\mathop{N{{H}_{4}}Cl}}\,\]. With Nessler’s reagents, it gives brown ppt.
\[\underset{Nessler's\ \,reagent}{\mathop{2{{K}_{2}}\left[ Hg{{I}_{4}} \right]}}\,\ +N{{H}_{3}}+KOH\to \underset{\underset{(Brown\,ppt)}{\mathop{Iodine\,\text{of M}illon's\,base}}\,}{\mathop{N{{H}_{2}}HgOHgI}}\,+7KI+2{{H}_{2}}O\]
It gives deep blue colour with \[CuS{{O}_{4}}\] solution, \[CuS{{O}_{4}}+4N{{H}_{3}}\to \underset{Deep\,blue}{\mathop{\left[ Cu{{(N{{H}_{3}})}_{4}} \right]S{{O}_{4}}}}\,\]. \[N{{H}_{3}}\] dissolves in water to give \[N{{H}_{4}}OH,\] which being basic, turns red litmus blue, \[N{{H}_{3}}+{{H}_{2}}O\to N{{H}_{4}}OH\]\[\rightleftharpoons \]\[NH_{4}^{+}+O{{H}^{-}}\].
(vii) Tests for HCl gas : It is colourless gas with a pungent irritating smell. It turns moist blue litmus paper red i.e., it is acidic in nature. It gives white ppt. with \[AgN{{O}_{3}}\] solution. This white ppt. is soluble in \[N{{H}_{4}}OH.\] \[HCl+AgN{{O}_{3}}\to \underset{White\,ppt.}{\mathop{AgCl}}\,+HN{{O}_{3}}\]; \[AgCl+2N{{H}_{4}}OH\to \underset{\text{Soluble}}{\mathop{\left[ Ag{{(N{{H}_{3}})}_{2}} \right]}}\,\ +2{{H}_{2}}O\].
(viii) Test for \[C{{H}_{3}}COOH\] vapours : These vapours are colourless with a vinegar like smell.
(2) Coloured gases
(i) Tests for \[\mathbf{C}{{\mathbf{l}}_{\mathbf{2}}}\]: It is a greenish yellow gas with a pungent smell. In small quantity it appears almost colourless. It bleaches a moist litmus paper, \[C{{l}_{2}}+{{H}_{2}}O\to 2HCl+\left[ O \right]\]; \[Colour+\left[ O \right]\to Colourless.\] Blue litmus paper first turns red and then becomes colourless.
(ii) Tests for \[\mathbf{B}{{\mathbf{r}}_{\mathbf{2}}}\] : Brown vapours with a pungent smell. It turns moist starch paper yellow.
(iii) Tests for \[{{\mathbf{I}}_{\mathbf{2}}}\] : Violet vapours with a pungent smell. It turns moist starch paper blue.
(iv) Tests for \[\mathbf{N}{{\mathbf{O}}_{\mathbf{2}}}\]: Brown coloured pungent smelling gas. It turns moist starch KI paper blue
\[2KI+2N{{O}_{2}}\to 2KN{{O}_{2}}+{{I}_{2}}\]; \[{{I}_{2}}+Starch\to Blue\,colour.\]
It turns ferrous sulphate solution black,
\[3FeS{{O}_{4}}+N{{O}_{2}}+{{H}_{2}}S{{O}_{4}}\to F{{e}_{2}}{{(S{{O}_{4}})}_{3}}+\underset{Black\,brown}{\mathop{FeS{{O}_{4}}.\,NO}}\,+{{H}_{2}}O\]
General characteristics of Halo-Alkenes (1) Organic compounds in which halogen atom \[(F,Cl,Br,I)\] is directly linked with saturated carbon atom are known as halo-alkanes. General formula is \[{{C}_{n}}{{H}_{2n+2-m}}{{X}_{m}}\] (\[X=F,Cl,Br,I\]) and \[m=\text{no}\text{.}\]of halogen atom; \[n=\text{no}\text{.}\]of carbon atoms. (2) Depending on the number of halogen atoms present in the halogen derivative, these are termed as mono-, di-, tri-, tetra-, and polyhalogen derivatives. \[\left[ \underset{\text{Methane}}{\mathop{C{{H}_{4}}}}\,\underset{+X}{\mathop{\xrightarrow{-H}}}\,\underset{\text{Mono}}{\mathop{C{{H}_{3}}-X}}\,\underset{+X}{\mathop{\xrightarrow{-H}}}\,\underset{\text{Di}}{\mathop{C{{H}_{2}}-{{X}_{2}}}}\,\underset{+X}{\mathop{\xrightarrow{-H}}}\,\underset{\text{Tri}}{\mathop{CH-{{X}_{3}}}}\,\underset{+X}{\mathop{\xrightarrow{-H}}}\,\underset{\text{Tetra}}{\mathop{C-{{X}_{4}}}}\, \right]\] (i) Monohalogen derivatives are termed as alkyl halides. Example: \[\underset{\text{Methyl}\,\text{chloride}}{\mathop{C{{H}_{3}}Cl}}\,\] \[\underset{\text{Ethyl}\,\text{bromide}}{\mathop{{{C}_{2}}{{H}_{5}}Br}}\,\] \[\underset{\text{Propyl}\,\text{iodide}}{\mathop{{{C}_{3}}{{H}_{7}}I}}\,\] Monohalogen derivatives or alkyl halides are classified as primary (1°), secondary (2°) or tertiary (3°) depending upon whether the halogen atom is attached to primary, secondary or tertiary carbon atoms. 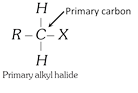 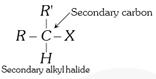 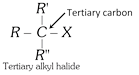 (ii) The dihalogen derivatives are mainly of three types (a) Gem-dihalides: In these derivatives both the halogen atoms are attached to the same carbon atom. These are also called alkylidene halides. (ii) The dihalogen derivatives are mainly of three types (a) Gem-dihalides: In these derivatives both the halogen atoms are attached to the same carbon atom. These are also called alkylidene halides. 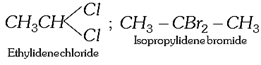 (b) Vic-dihalides : In these derivatives, the halogen atoms are attached to adjacent (Vicinal) carbon atoms. These are also termed as alkylene halides. \[\underset{\text{Ethylene}\,\text{chloride}}{\mathop{C{{H}_{2}}Cl.C{{H}_{2}}Cl}}\,\] ; \[\underset{\text{Propylene}\,\text{chloride}}{\mathop{C{{H}_{3}}CHCl.C{{H}_{2}}Cl}}\,\] (c) a-w halides (Terminal dihalides): In these derivatives, the halogen atoms are attached to terminal carbon atoms. These are also called polymethylene halides. \[\underset{\text{Trimethylene bromide }}{\mathop{C{{H}_{2}}BrC{{H}_{2}}C{{H}_{2}}Br}}\,\] ; \[\underset{\text{Tetra-methylene chloride}}{\mathop{Cl-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-Cl}}\,\] (iii) The tri-halogen derivatives are termed as halo-forms Example: \[\underset{\text{Chloroform}}{\mathop{CHC{{l}_{3}}}}\,\]; \[\underset{\text{Bromoform}}{\mathop{CHB{{r}_{3}}}}\,\]; \[\underset{I\text{odoform}}{\mathop{CH{{I}_{3}}}}\,\] (iv) In tetra-halogen derivatives all the four halogen atoms are attached to the same carbon atom in derivatives of methane. Example: \[\underset{\text{Carbon}\,\text{tetrachloride}}{\mathop{CC{{l}_{4}}}}\,\]; \[\underset{\text{Carbon}\,\text{tetrabromide}}{\mathop{CB{{r}_{4}}}}\,\] In other derivatives, the four halogen atoms are attached to different carbon atoms, e.g.,\[\underset{\begin{smallmatrix} \text{Acetylene tetrachloride or} \\ \text{1,1,2,2-tetrachloroethane} \end{smallmatrix}}{\mathop{\underset{CHC{{l}_{2}}}{\mathop{\underset{|\ \ \ \ \ \ \ \ \ \ \ }{\mathop{CHC{{l}_{2}}}}\,}}\,}}\,\] (3) The common and IUPAC names of some halogen derivatives are listed here. (b) Vic-dihalides : In these derivatives, the halogen atoms are attached to adjacent (Vicinal) carbon atoms. These are also termed as alkylene halides. \[\underset{\text{Ethylene}\,\text{chloride}}{\mathop{C{{H}_{2}}Cl.C{{H}_{2}}Cl}}\,\] ; \[\underset{\text{Propylene}\,\text{chloride}}{\mathop{C{{H}_{3}}CHCl.C{{H}_{2}}Cl}}\,\] (c) a-w halides (Terminal dihalides): In these derivatives, the halogen atoms are attached to terminal carbon atoms. These are also called polymethylene halides. \[\underset{\text{Trimethylene bromide }}{\mathop{C{{H}_{2}}BrC{{H}_{2}}C{{H}_{2}}Br}}\,\] ; \[\underset{\text{Tetra-methylene chloride}}{\mathop{Cl-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-Cl}}\,\] (iii) The tri-halogen derivatives are termed as halo-forms Example: \[\underset{\text{Chloroform}}{\mathop{CHC{{l}_{3}}}}\,\]; \[\underset{\text{Bromoform}}{\mathop{CHB{{r}_{3}}}}\,\]; \[\underset{I\text{odoform}}{\mathop{CH{{I}_{3}}}}\,\] (iv) In tetra-halogen derivatives all the four halogen atoms are attached to the same carbon atom in derivatives of methane. Example: \[\underset{\text{Carbon}\,\text{tetrachloride}}{\mathop{CC{{l}_{4}}}}\,\]; \[\underset{\text{Carbon}\,\text{tetrabromide}}{\mathop{CB{{r}_{4}}}}\,\] In other derivatives, the four halogen atoms are attached to different carbon atoms, e.g.,\[\underset{\begin{smallmatrix} \text{Acetylene tetrachloride or} \\ \text{1,1,2,2-tetrachloroethane} \end{smallmatrix}}{\mathop{\underset{CHC{{l}_{2}}}{\mathop{\underset{|\ \ \ \ \ \ \ \ \ \ \ }{\mathop{CHC{{l}_{2}}}}\,}}\,}}\,\] (3) The common and IUPAC names of some halogen derivatives are listed here.
|