| Elements | % of dry weight |
| Macronutrients | |
| Carbon | 45 |
| Oxygen | 45 |
| more...
Competitive inhibition : Substances (inhibitors) which are structurally similar to the substrates and competes for the active site of the enzyme are known as competitive inhibitors. Usually such inhibitors show a close structural resemblance to the substrates to the enzyme they inhibit. In such a case, inspite of enzyme substrate complex, enzyme inhibitor complex is formed and enzyme activity is inhibited.
\[\underset{\text{Enzyme}}{\mathop{\text{E}}}\,+\underset{\text{inhibitor}}{\mathop{\text{I}}}\,\to \underset{\text{Enzyme}-\text{inhibitor}\,\text{complex(EI)}}{\mathop{\text{EI}}}\,\]
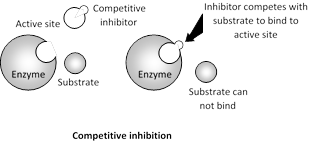 The concentration of \[EI\]complex depends on the concentration of free inhibitor. Because \[EI\]complex readily dissociates, the empty active sites are then available for substrate binding. The effect of a competitive inhibitor on activity is reversed by increasing the concentration of substrate. In it \[{{V}_{\max }}\] remain constant and Km increases.
A classic example of competitive inhibition is succinic acid dehydrogenase which oxidises succinic acid to fumaric acid. If concentration of malonic acid, is added, the activity of succinic dehydrogenase decreases rapidly. Hence malonic acid acts as a competitive inhibitor since it has structural resemblance to succinc acid.
The competitive inhibition can be reversed by increasing the concentration of the substrate. Competitive inhibitors are used in control of bacterial pathogens.
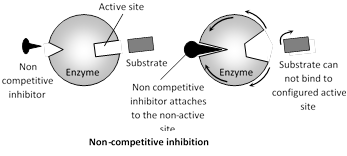
Non-competitive inhibition : These substances (poisons) do not combine with active sites but attach somewhere else and destroy the activity of enzyme.
Both EI and ES complexes are formed. Inhibitor binding alters the three dimensional configuration of the enzyme and thus blocks the reaction. Non competitive inhibitor do not competes directly with the substrate for binding to the enzyme. In it \[{{V}_{\max }}\] in lowered and Km is changed.
The non-competitive inhibition can not be reversed by increasing the concentration of the substrate i.e., irreversible. e.g., cyanide inhibits the mitochondrial enzyme cytochrome oxidase which is essential for cellular respiration. This kills the animals.
More AMP is a non competitive inhibitor of fructose biphosphate phosphatase, the enzyme that catalyzes the conversion of fructose 1, 6 biphosphate to fructose 6 phosphate.
The concentration of \[EI\]complex depends on the concentration of free inhibitor. Because \[EI\]complex readily dissociates, the empty active sites are then available for substrate binding. The effect of a competitive inhibitor on activity is reversed by increasing the concentration of substrate. In it \[{{V}_{\max }}\] remain constant and Km increases.
A classic example of competitive inhibition is succinic acid dehydrogenase which oxidises succinic acid to fumaric acid. If concentration of malonic acid, is added, the activity of succinic dehydrogenase decreases rapidly. Hence malonic acid acts as a competitive inhibitor since it has structural resemblance to succinc acid.
The competitive inhibition can be reversed by increasing the concentration of the substrate. Competitive inhibitors are used in control of bacterial pathogens.
Non-competitive inhibition : These substances (poisons) do not combine with active sites but attach somewhere else and destroy the activity of enzyme.
Both EI and ES complexes are formed. Inhibitor binding alters the three dimensional configuration of the enzyme and thus blocks the reaction. Non competitive inhibitor do not competes directly with the substrate for binding to the enzyme. In it \[{{V}_{\max }}\] in lowered and Km is changed.
The non-competitive inhibition can not be reversed by increasing the concentration of the substrate i.e., irreversible. e.g., cyanide inhibits the mitochondrial enzyme cytochrome oxidase which is essential for cellular respiration. This kills the animals.
More AMP is a non competitive inhibitor of fructose biphosphate phosphatase, the enzyme that catalyzes the conversion of fructose 1, 6 biphosphate to fructose 6 phosphate.
 Feedback inhibition : In number of cases, accumulation of the final product of the reaction is capable of inhibiting the first step of reaction.
Feedback inhibition : In number of cases, accumulation of the final product of the reaction is capable of inhibiting the first step of reaction.
Substrate concentration : If there are more enzyme molecules than substrate molecules, a progressive increase in the substrate molecules increases the velocity of their conversion to products. However, eventually the rate of reaction reaches the maximum. At this stage the active sites of all the available enzyme molecules are occupied by the substrate molecules. Therefore, the substrate molecules occupy the active sites vacated by the products and cannot increase the rate of reaction further.
Enzyme concentration : The rate of reaction is directly proportional to enzyme concentration. An increase in enzyme concentration will cause a rise in the rate of reaction up to a point and them the rate of reaction will be constant. Increasing the enzyme concentration increases the number of available active sites.
Product concentration : Accumulation of the product of enzyme reaction lowers the enzyme activity. Enzyme molecules must be freed to combine with more substrate molecules. Normally the product are quickly removed from the site of formation and the reaction does not suffer.
Hydrogen ion concentration (pH) : Some enzyme act best in an acid medium, other in an alkline medium, for every enzyme there is an optimum pH where its action is maximum e.g., 2 for pepsin, 6.8 for salivary amylase, 8.5 for trypsin. Most enzyme show maximum activity in a pH range of about 6.0 to 7.5 i.e., near neutral pH (endoenzymes). A shift to the alkaline or acid side rapidly decreases the enzyme activity and finally stops it altogether. This is due to denaturation of enzyme molecule i.e., change in its physical structure.
Temperature : Within certain limits \[(5-40{}^\circ C)\] the rate of an enzyme catalyzed reaction increases as the temperature increases. The \[{{Q}_{10}}\] of most enzymatic reactions is 2, i.e., every 10°C rise in temperature doubles the rate of reaction. Most enzymes show maximum activity in a temperature range of 25 to 40°C. Beyond this temperature, there is sharp fall in the rate of reaction.
Modification in the physical form of the enzyme results in the loss of its catalytic activity. This change in structure is called denaturation of protein. This is the permanent change, and the denatured enzyme protein remains inactive even if the temperature is then brought down. The enzymes are not destroyed by freezing, and regain their lost activity if the temperature is raised to normal.
Deep freezing of food for preserving them for long periods is done not only to prevent the growth and multiplication of microorganisms but also to inactivate enzymes. It makes impossible for the microorganisms to digest the food.
Enzyme inhibitors : Certain chemical compounds inhibit activity of enzyme molecules either permanently or temporarily. Thus, di-isopropyl flurophosphate (DFP) inhibits the action of various enzymes catalysing hydrolysis of ester linkage. Inhibition is permanent or irreversible.
Poisons and Radiation : Poisons such as cyanide and radiation destroy the tertiary structure of the enzymes, making them ineffective.
Enzymes (Gk. en = in; zyme = yeast) are proteinaceous substances which are capable of catalysing chemical reactions of biological origins without themselves undergoing any change. Enzymes are biocatalysts. An enzyme may be defined as "a protein that enhances the rate of biochemical reactions but does not affect the nature of final product". Like the catalyst the enzymes regulate the speed and specificity of a reaction, but unlike the catalyst they are produced by living cells only. All components of cell including cell wall and cell membrane have enzymes.
Maximum enzymes (70%) in the cell are found in mitochondrion. Enzymes are also called 'biological middle man'. The study of the composition and function of the enzyme is known as enzymology.
The term enzyme (meaning in yeast) was used by Willy Kuhne (1878) while working on fermentation. At that time living cells of yeast were thought to be essential for fermentation of sugar. Edward Buchner (1897), a German chemist proved that extract zymase, obtained from yeast cells, has the power of fermenting sugar (alcoholic fermentation). Zymase is complex of enzymes (Buchner isolated enzyme for the first time).
Later J.B. Sumner (1926) prepared a pure crystalline form of urease enzyme from Jack Bean (Canavalia ensiformis) and suggested that enzymes are proteins. Northrop and Kunitz prepared crystals of pepsin, trypsin and chymotrypsin Arber and Nathans got noble prize in 1978 for the discovery of restriction endonucleases which break both strands of DNA at specific sites and produce sticky ends. These enzymes are used as microscissors in genetic engineering.
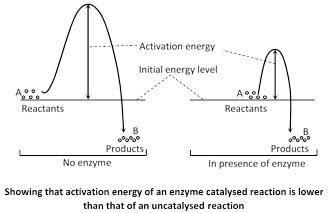
Energy is required to bring the inert molecules into the activated state. The amount of energy required to raise the energy of molecules at which chemical reaction can occur is called activation energy. Enzymes act by decreasing the activation energy so that the number of activated molecules is increased at lower energy levels. If the activation energy required for the formation of the enzyme-substrate complex is low, many more molecules can participate in the reaction than would be the case if the enzyme were absent.
In 1913 Michaelis and Menten proposed that for a catalylic reaction to occur it is necessary that enzyme and substrate bind together to form an enzyme substrate complex.
\[\underset{(Enzyme)}{\mathop{E}}\,+\underset{(Substrate)}{\mathop{S}}\,\to \underset{(Enzyme-substrate\text{ }Complex)}{\mathop{E-S\text{ }Complex}}\,\]
\[E-S\text{ }Complex\to \underset{(Enzyme)}{\mathop{E}}\,+\underset{(\Pr oduct)}{\mathop{P}}\,\]
It is amazing that the enzyme-substrate complex breaks up into chemical products different from those, which participated in its formation (i.e., substrates). On the surface of each enzyme there are many specific sites for binding substrate molecules called active sites or catalytic sites.
There are two views regarding the mode of enzyme action :
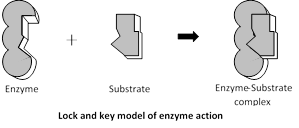
Lock and Key hypothesis : The hypothesis was put forward by Emil Fisher (1894). According to this hypothesis the enzyme and its substrate have a complementary shape. The specific substrate molecules are bound to a specific site of the enzyme molecule.
The theory can be explained easily by the fact that a particular lock can be opened by a particular key specially designed to open it. Similarly enzymes have specific sites where a particular substrate can only be attached. The lock and key model accounts for enzyme specificity.
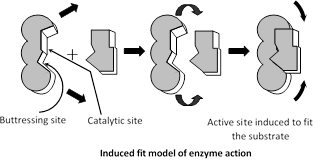
 Induced fit hypothesis : This hypothesis was proposed by Daniel, E. Koshland (1959).
According to this view, active site is not rigid but static and it has two groups - buttressing group and catalytic group. Initially substrate bind to the buttressing group which induces the catalytic group to fit the substrate and catalytic group weakes the bonds of reactant or substrate by electrophilic and nucleophilic forces.
Induced fit hypothesis : This hypothesis was proposed by Daniel, E. Koshland (1959).
According to this view, active site is not rigid but static and it has two groups - buttressing group and catalytic group. Initially substrate bind to the buttressing group which induces the catalytic group to fit the substrate and catalytic group weakes the bonds of reactant or substrate by electrophilic and nucleophilic forces.
Mostly enzymes are proteinaceous in nature. With some exception all enzymes are proteins but all proteins are not enzymes. Enzymatic protein consist of 20 amino acids. The polypeptide chain or chains of an enzyme show tertiary structure. Their tertiary structure is very specific and important for their biological activity. Loss of tertiary structure renders the enzymic activity.
Some enzymes like pepsin, amylase, urease, etc., are exclusively made up of protein i.e., simple proteins. But most of the other enzymes have a protein and a non-protein component, both of which are essential for enzyme activity. The protein component of such enzymes is known as apoenzyme whereas the non-protein component is called cofactor or prosthetic group. The apoenzyme and prosthetic group together form a complete enzyme called holoenzyme.
Activity of enzyme is due to co-factor, which can be separated by dialysis. co-factor is small, heat stable and may be organic or inorganic in nature.
Three types of cofactors may be identified. Prosthetic group, coenzyme and metal ions.
Prosthetic group : Prosthetic groups are organic compounds distinguished from other cofactors in that they are permanently bound to the apoenzyme, e.g., in peroxisomal enzymes peroxidase and catalase which catalyzes breakdown of hydrogen peroxide to water and oxygen.
Coenzymes : Fritz Lipmann discovered coenzymes. Coenzymes are also organic compounds but their association with the apoenzyme is transient, usually occurring only during the course of catalysis.
In general coenzymes not only assist enzymes in the cleavage of the substrate but also serve as temporary acceptor for one of the product of the reaction. The essential chemical component of many coenzymes are vitamins, e.g., coenzyme nicotinamide adenine dinucleotide (NAD), nicotinamide adenine dinucleotide phosphate (NADP) contains the vitamin niacin, coenzyme A contains pantothenic acid, flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD) contains riboflavin (Vitamin\[{{B}_{2}}\]), and thiamine pyrophosphate (TPP) contains thiamine (Vitamin \[{{B}_{1}}\]).
Metal ions : A number of enzymes require metal ions for their activity. The metal ions form coordination bonds with specific side chains at the active site and at the same time form one or more coordination bonds with the substrate. The latter assist in the polarization of substrate bonds to be cleaved by the enzyme. The common metal ions are \[Z{{n}^{++}},C{{u}^{++}},M{{g}^{++}}.\]
Inorganic part of enzyme acts as prosthetic group in few enzymes they are called activators. These activators are generally metals. Hence these enzymes are called Metalloenzyme such as :
Enzymes activators
| |