Category : 11th Class
Competitive inhibition : Substances (inhibitors) which are structurally similar to the substrates and competes for the active site of the enzyme are known as competitive inhibitors. Usually such inhibitors show a close structural resemblance to the substrates to the enzyme they inhibit. In such a case, inspite of enzyme substrate complex, enzyme inhibitor complex is formed and enzyme activity is inhibited.
\[\underset{\text{Enzyme}}{\mathop{\text{E}}}\,+\underset{\text{inhibitor}}{\mathop{\text{I}}}\,\to \underset{\text{Enzyme}-\text{inhibitor}\,\text{complex(EI)}}{\mathop{\text{EI}}}\,\]
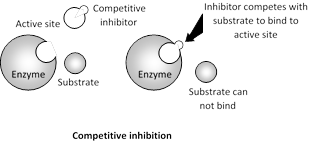
The concentration of \[EI\]complex depends on the concentration of free inhibitor. Because \[EI\]complex readily dissociates, the empty active sites are then available for substrate binding. The effect of a competitive inhibitor on activity is reversed by increasing the concentration of substrate. In it \[{{V}_{\max }}\] remain constant and Km increases.
A classic example of competitive inhibition is succinic acid dehydrogenase which oxidises succinic acid to fumaric acid. If concentration of malonic acid, is added, the activity of succinic dehydrogenase decreases rapidly. Hence malonic acid acts as a competitive inhibitor since it has structural resemblance to succinc acid.
The competitive inhibition can be reversed by increasing the concentration of the substrate. Competitive inhibitors are used in control of bacterial pathogens.
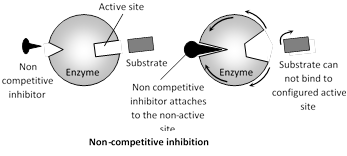
Non-competitive inhibition : These substances (poisons) do not combine with active sites but attach somewhere else and destroy the activity of enzyme.
Both EI and ES complexes are formed. Inhibitor binding alters the three dimensional configuration of the enzyme and thus blocks the reaction. Non competitive inhibitor do not competes directly with the substrate for binding to the enzyme. In it \[{{V}_{\max }}\] in lowered and Km is changed.
The non-competitive inhibition can not be reversed by increasing the concentration of the substrate i.e., irreversible. e.g., cyanide inhibits the mitochondrial enzyme cytochrome oxidase which is essential for cellular respiration. This kills the animals.
More AMP is a non competitive inhibitor of fructose biphosphate phosphatase, the enzyme that catalyzes the conversion of fructose 1, 6 biphosphate to fructose 6 phosphate.
Feedback inhibition : In number of cases, accumulation of the final product of the reaction is capable of inhibiting the first step of reaction.
![]()
The product P checks the activity of enzyme which converts A into B. It is quite useful mechanism because it checks the accumulation of products.
The phenomenon in which the end product of a metabolic pathway can regulate its own production by inhibition of the sort is called feed back inhibition or negative feed back inhibition. This type of inhibition can be shown in Escherichia coli bacterium which synthesises the amino acid isoleucine from a substrate threonine by a series of intermediate reactions (i.e., \[\alpha \]ketobutyrate threonine deaminase, \[\alpha \]Aceto hydroxy butyrate, \[\alpha \]keto\[\beta \]methyl valerate etc).
When isoleucine accumulates in amounts more than required, it stops its own production by inhibiting the activity of the enzyme. Threonine deaminase which catalyzes the first reaction of the series. This type of metabolic control in which the first enzyme of a series is inhibited by the end product, is known as end product inhibition.
Allosteric inhibition (Modulation) : Allosteric literally means 'another place'. Still other inhibitors join an enzyme at a specific site and change the form of the active site meant for the substrate. These inhibitors are known as modifiers or modulators and the sites where they fit in are called allosteric sites. Modulators are of two types-positive (activators) and negative (inhibitors).
Change of active site form prevent the binding of substrate to the enzyme and stops the reaction. The process is called allostery or allosteric inhibition, The enzyme with allosteric sites are called allosteric enzymes. Jacob and Monod have termed this phenomenon as allosteric transition.
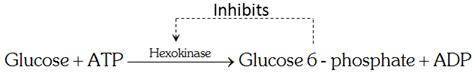
An example of allosteric enzyme inhibition is hexokinase that converts glucose to glucose 6-phosphate. Glucose 6-phosphate causes allosteric inhibition of hexokinase. This is called feedback allosteric inhibition.
You need to login to perform this action.
You will be redirected in
3 sec
