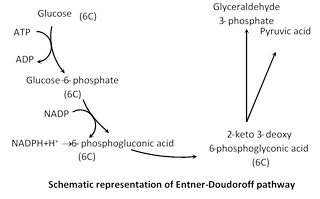
 (2) Pentose phosphate pathway
(i) Discovery : It is also called as Hexose monophosphate (HMP) shunt or Warburg Dickens pathway or direct oxidation pathway. It provides as alternative pathway for breakdown of glucose which is independent of EMP pathway (glycolysis) and Krebs cycle. Its existence was suggested for the first time by Warburg et al. (1935) and Dickens (1938). Most of the reaction of this cycle were described by Horecker et al. (1951) and Racker (1954).
(ii) Occurrence : Pentose phosphate pathway that exists in many organisms. This pathway takes place in the cytoplasm and requires oxygen for its entire operation.
(iii) Description : There are two types of evidences is support of the existence of such an alternative pathway-works on the inhibiting action of malonic acid on the Krebs cycle and studies with the radioactive \[({{C}^{14}}).\]
Twelve molecules of \[NAD{{H}_{2}}\] formed in the reaction can be oxidised back to 12 NADP with the help of the cytochrome system and oxygen of the air.
\[12\text{ NADP}{{\text{H}}_{\text{2}}}+6{{O}_{2}}\underset{\text{System}}{\mathop{\xrightarrow{\text{Cytochrome}}}}\,\text{12}{{\text{H}}_{\text{2}}}\text{O}+\text{12NADP}\]
In this electron transfer process, 36 molecules of ATP are synthesized.
(2) Pentose phosphate pathway
(i) Discovery : It is also called as Hexose monophosphate (HMP) shunt or Warburg Dickens pathway or direct oxidation pathway. It provides as alternative pathway for breakdown of glucose which is independent of EMP pathway (glycolysis) and Krebs cycle. Its existence was suggested for the first time by Warburg et al. (1935) and Dickens (1938). Most of the reaction of this cycle were described by Horecker et al. (1951) and Racker (1954).
(ii) Occurrence : Pentose phosphate pathway that exists in many organisms. This pathway takes place in the cytoplasm and requires oxygen for its entire operation.
(iii) Description : There are two types of evidences is support of the existence of such an alternative pathway-works on the inhibiting action of malonic acid on the Krebs cycle and studies with the radioactive \[({{C}^{14}}).\]
Twelve molecules of \[NAD{{H}_{2}}\] formed in the reaction can be oxidised back to 12 NADP with the help of the cytochrome system and oxygen of the air.
\[12\text{ NADP}{{\text{H}}_{\text{2}}}+6{{O}_{2}}\underset{\text{System}}{\mathop{\xrightarrow{\text{Cytochrome}}}}\,\text{12}{{\text{H}}_{\text{2}}}\text{O}+\text{12NADP}\]
In this electron transfer process, 36 molecules of ATP are synthesized.
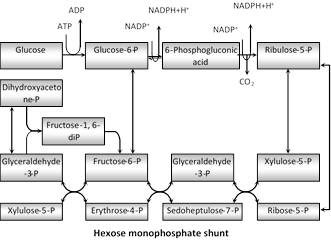 (iv) Significance of PPP
(a) It is the only pathway of carbohydrate oxidation that gives \[NADP{{H}_{2}},\]Which is needed for synthetic action like synthesis of fatty acid (in adipose tissues) and amino acids (in liver).
(b) It synthesizes 3C-glyceraldehyde-3-P, 3C-dihydroxy acetone phosphate, 4C-erythrose-4-P, 5C-ribulose phosphate, 5C-xylulose phosphate, 5C-ribose phosphate, 6 C-Fructose 6-phosphate, 7C-sedoheptulose-7-phosphate.
(c) It is the major pathway by which necessary ribose and deoxyribose are supplied in the biosynthesis of nucleotides and nucleic acid.
(d) Erythrose 4 phosphate for the synthesis of lignin, oxine, anthocyanine and aromatic amino acid (phenylalanine, tyrosine, and tryptophan).
(e) Young growing tissues appears to use to the Krebs cycle as the predominant pathway for glucose oxidation, while aerial parts of the plants and other tissues seem to utilise the PPP as well as the Krebs cycle.
(f) It gives \[6C{{O}_{2}},\]required for photosynthesis.
(g) Ribulose five phosphate is used in photosynthesis to produce RuBP which act as primary \[C{{O}_{2}}\]acceptor in \[{{C}_{3}}\]cycle.
(3) Cyanide resistant pathway : Cyanide-resistant respiration seems to be widespread in higher plant tissues. Cyanide prevents flow of electron from Cyt \[{{a}_{3}}\] to oxygen, so called ETC inhibitor. In these plant tissues resistance is due to, a branch point in the ETS preceeding the highly cyanide-sensitive cytochromes. The tissues lacking this branch point, or alternate pathway and blockage of cytochromes by cyanide, inhibits the electron flow.
Significance
(i) The role of alternative pathway is that it may provide a means for the continued oxidation of NADH and operation of the tricarboxylic acid more...
(iv) Significance of PPP
(a) It is the only pathway of carbohydrate oxidation that gives \[NADP{{H}_{2}},\]Which is needed for synthetic action like synthesis of fatty acid (in adipose tissues) and amino acids (in liver).
(b) It synthesizes 3C-glyceraldehyde-3-P, 3C-dihydroxy acetone phosphate, 4C-erythrose-4-P, 5C-ribulose phosphate, 5C-xylulose phosphate, 5C-ribose phosphate, 6 C-Fructose 6-phosphate, 7C-sedoheptulose-7-phosphate.
(c) It is the major pathway by which necessary ribose and deoxyribose are supplied in the biosynthesis of nucleotides and nucleic acid.
(d) Erythrose 4 phosphate for the synthesis of lignin, oxine, anthocyanine and aromatic amino acid (phenylalanine, tyrosine, and tryptophan).
(e) Young growing tissues appears to use to the Krebs cycle as the predominant pathway for glucose oxidation, while aerial parts of the plants and other tissues seem to utilise the PPP as well as the Krebs cycle.
(f) It gives \[6C{{O}_{2}},\]required for photosynthesis.
(g) Ribulose five phosphate is used in photosynthesis to produce RuBP which act as primary \[C{{O}_{2}}\]acceptor in \[{{C}_{3}}\]cycle.
(3) Cyanide resistant pathway : Cyanide-resistant respiration seems to be widespread in higher plant tissues. Cyanide prevents flow of electron from Cyt \[{{a}_{3}}\] to oxygen, so called ETC inhibitor. In these plant tissues resistance is due to, a branch point in the ETS preceeding the highly cyanide-sensitive cytochromes. The tissues lacking this branch point, or alternate pathway and blockage of cytochromes by cyanide, inhibits the electron flow.
Significance
(i) The role of alternative pathway is that it may provide a means for the continued oxidation of NADH and operation of the tricarboxylic acid more... You need to login to perform this action.
You will be redirected in
3 sec
