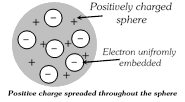
 (2) This model failed to explain the line spectrum of an element and the scattering experiment of Rutherford.
(2) This model failed to explain the line spectrum of an element and the scattering experiment of Rutherford.
 Examples : \[CO,\text{ }{{N}_{2}}O,\text{ }{{H}_{2}}{{O}_{2}},\text{ }{{N}_{2}}{{O}_{3}},\text{ }{{N}_{2}}{{O}_{4}},\text{ }{{N}_{2}}{{O}_{5}},\text{ }HN{{O}_{3}},\] \[NO_{3}^{-}\], \[S{{O}_{2}},\text{ }S{{O}_{3}},\text{ }{{H}_{2}}S{{O}_{4}},\] \[SO_{4}^{2-},SO_{2}^{2-},\] \[{{H}_{3}}P{{O}_{4}},\]\[{{H}_{4}}{{P}_{2}}{{O}_{7}},\] \[{{H}_{3}}P{{O}_{3}},A{{l}_{2}}C{{l}_{6}}(\text{Anhydrous),}{{O}_{3}},S{{O}_{2}}C{{l}_{2}},SOC{{l}_{2}},HI{{O}_{3}},HCl{{O}_{4}},\]\[HCl{{O}_{3}},C{{H}_{3}}NC,{{N}_{2}}H_{5}^{+}\], \[C{{H}_{3}}N{{O}_{2}},NH_{4}^{+},\ {{[Cu{{(N{{H}_{3}})}_{4}}]}^{2+}}\] etc.
Characteristics of co-ordinate covalent compound
(1) Their melting and boiling points are higher than purely covalent compounds more...
Examples : \[CO,\text{ }{{N}_{2}}O,\text{ }{{H}_{2}}{{O}_{2}},\text{ }{{N}_{2}}{{O}_{3}},\text{ }{{N}_{2}}{{O}_{4}},\text{ }{{N}_{2}}{{O}_{5}},\text{ }HN{{O}_{3}},\] \[NO_{3}^{-}\], \[S{{O}_{2}},\text{ }S{{O}_{3}},\text{ }{{H}_{2}}S{{O}_{4}},\] \[SO_{4}^{2-},SO_{2}^{2-},\] \[{{H}_{3}}P{{O}_{4}},\]\[{{H}_{4}}{{P}_{2}}{{O}_{7}},\] \[{{H}_{3}}P{{O}_{3}},A{{l}_{2}}C{{l}_{6}}(\text{Anhydrous),}{{O}_{3}},S{{O}_{2}}C{{l}_{2}},SOC{{l}_{2}},HI{{O}_{3}},HCl{{O}_{4}},\]\[HCl{{O}_{3}},C{{H}_{3}}NC,{{N}_{2}}H_{5}^{+}\], \[C{{H}_{3}}N{{O}_{2}},NH_{4}^{+},\ {{[Cu{{(N{{H}_{3}})}_{4}}]}^{2+}}\] etc.
Characteristics of co-ordinate covalent compound
(1) Their melting and boiling points are higher than purely covalent compounds more...
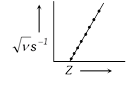
 (ii) It was determined by Moseley
as,
\[\sqrt{\nu }=a(Z-b)\] or \[aZ-ab\]
Where, \[\nu =X-\]ray?s frequency
Z= atomic number of the metal \[a\And
b\] are constant.
(iii) Atomic number = Number of
positive charge on nucleus = Number of protons in nucleus = Number of electrons
in nutral atom.
(iv) Two different elements can
never have identical atomic number.
(2) Mass number
Mass number (A) = Number of protons or
Atomic number (Z) + Number of neutrons or Number of neutrons = A ?
Z .
(i) Since mass of
a proton or a neutron is not a whole number (on atomic weight scale), weight is
not necessarily a whole number.
(ii) The atom of an element X having
mass number (A) and more...
(ii) It was determined by Moseley
as,
\[\sqrt{\nu }=a(Z-b)\] or \[aZ-ab\]
Where, \[\nu =X-\]ray?s frequency
Z= atomic number of the metal \[a\And
b\] are constant.
(iii) Atomic number = Number of
positive charge on nucleus = Number of protons in nucleus = Number of electrons
in nutral atom.
(iv) Two different elements can
never have identical atomic number.
(2) Mass number
Mass number (A) = Number of protons or
Atomic number (Z) + Number of neutrons or Number of neutrons = A ?
Z .
(i) Since mass of
a proton or a neutron is not a whole number (on atomic weight scale), weight is
not necessarily a whole number.
(ii) The atom of an element X having
mass number (A) and more...
 (ii) It was determined by Moseley as,
\[\sqrt{\nu }=a(Z-b)\] or \[aZ-ab\]
Where, \[\nu =X-\]ray?s frequency
Z= atomic number of the metal \[a\And b\] are constant.
(iii) Atomic number = Number of positive charge on nucleus = Number of protons in nucleus = Number of electrons in nutral atom.
(iv) Two different elements can never have identical atomic number.
(2) Mass number Mass number (A) = Number of protons or Atomic number (Z) + Number of neutrons or Number of neutrons = A ? Z .
(i) Since mass of a proton or a neutron is not a whole number (on atomic weight scale), weight is not necessarily a whole number.
(ii) The atom of an element X having mass more...
(ii) It was determined by Moseley as,
\[\sqrt{\nu }=a(Z-b)\] or \[aZ-ab\]
Where, \[\nu =X-\]ray?s frequency
Z= atomic number of the metal \[a\And b\] are constant.
(iii) Atomic number = Number of positive charge on nucleus = Number of protons in nucleus = Number of electrons in nutral atom.
(iv) Two different elements can never have identical atomic number.
(2) Mass number Mass number (A) = Number of protons or Atomic number (Z) + Number of neutrons or Number of neutrons = A ? Z .
(i) Since mass of a proton or a neutron is not a whole number (on atomic weight scale), weight is not necessarily a whole number.
(ii) The atom of an element X having mass more...
 i.e.for any triangle the ratio of the sine of the angle containing the side to the length of the side is a constant. For a triangle whose three sides are in the same order we establish the Lami's theorem in the following manner. For the triangle shown
\[\overrightarrow{a}+\overrightarrow{b}+\overrightarrow{c}=\overrightarrow{0}\] [All three sides are taken in order] ?
(i) \[\Rightarrow \]\[\overrightarrow{a}+\overrightarrow{b}=-\overrightarrow{c}\] ?
(ii) Pre-multiplying both sides by \[\overrightarrow{a}\]\[\overrightarrow{a}\times (\overrightarrow{a}+\overrightarrow{b})=-\overrightarrow{a}\times \overrightarrow{c}\]
\[\Rightarrow \]\[\overrightarrow{0}+\overrightarrow{a}\times \overrightarrow{b}=-\overrightarrow{a}\times \overrightarrow{c}\]
\[\Rightarrow \,\,\,\,\overrightarrow{a}\times \overrightarrow{b}=\overrightarrow{c}\times \overrightarrow{a}\] ?(iii) Pre-multiplying both sides of
(ii) by \[\overrightarrow{b}\] \[\overrightarrow{b}\times (\overrightarrow{a}+\overrightarrow{b})=-\,\overrightarrow{b}\times \overrightarrow{c}\]
\[\Rightarrow \,\,\,\,\overrightarrow{b}\times \overrightarrow{a}+\overrightarrow{b}\times \overrightarrow{b}=-\overrightarrow{b}\times \overrightarrow{c}\]
\[\Rightarrow \,\,\,\,-\overrightarrow{a}\times \overrightarrow{b}=-\overrightarrow{b}\times \overrightarrow{c}\]\[\Rightarrow \,\,\,\overrightarrow{a}\times \overrightarrow{b}=\overrightarrow{b}\times \overrightarrow{c}\] ?
(iv) From (iii) and (iv), we get \[\overrightarrow{a}\times \overrightarrow{b}=\overrightarrow{b}\times \overrightarrow{c}=\overrightarrow{c}\times \overrightarrow{a}\] Taking magnitude, we get \[|\overrightarrow{a}\times \overrightarrow{b}|\,=\,|\overrightarrow{b}\times \overrightarrow{c}|\,=\,|\overrightarrow{c}\times \overrightarrow{a}|\] \[\Rightarrow \,\,\,ab\sin (180-\gamma )=bc\sin (180-\alpha )=ca\sin (180-\beta )\] more...
i.e.for any triangle the ratio of the sine of the angle containing the side to the length of the side is a constant. For a triangle whose three sides are in the same order we establish the Lami's theorem in the following manner. For the triangle shown
\[\overrightarrow{a}+\overrightarrow{b}+\overrightarrow{c}=\overrightarrow{0}\] [All three sides are taken in order] ?
(i) \[\Rightarrow \]\[\overrightarrow{a}+\overrightarrow{b}=-\overrightarrow{c}\] ?
(ii) Pre-multiplying both sides by \[\overrightarrow{a}\]\[\overrightarrow{a}\times (\overrightarrow{a}+\overrightarrow{b})=-\overrightarrow{a}\times \overrightarrow{c}\]
\[\Rightarrow \]\[\overrightarrow{0}+\overrightarrow{a}\times \overrightarrow{b}=-\overrightarrow{a}\times \overrightarrow{c}\]
\[\Rightarrow \,\,\,\,\overrightarrow{a}\times \overrightarrow{b}=\overrightarrow{c}\times \overrightarrow{a}\] ?(iii) Pre-multiplying both sides of
(ii) by \[\overrightarrow{b}\] \[\overrightarrow{b}\times (\overrightarrow{a}+\overrightarrow{b})=-\,\overrightarrow{b}\times \overrightarrow{c}\]
\[\Rightarrow \,\,\,\,\overrightarrow{b}\times \overrightarrow{a}+\overrightarrow{b}\times \overrightarrow{b}=-\overrightarrow{b}\times \overrightarrow{c}\]
\[\Rightarrow \,\,\,\,-\overrightarrow{a}\times \overrightarrow{b}=-\overrightarrow{b}\times \overrightarrow{c}\]\[\Rightarrow \,\,\,\overrightarrow{a}\times \overrightarrow{b}=\overrightarrow{b}\times \overrightarrow{c}\] ?
(iv) From (iii) and (iv), we get \[\overrightarrow{a}\times \overrightarrow{b}=\overrightarrow{b}\times \overrightarrow{c}=\overrightarrow{c}\times \overrightarrow{a}\] Taking magnitude, we get \[|\overrightarrow{a}\times \overrightarrow{b}|\,=\,|\overrightarrow{b}\times \overrightarrow{c}|\,=\,|\overrightarrow{c}\times \overrightarrow{a}|\] \[\Rightarrow \,\,\,ab\sin (180-\gamma )=bc\sin (180-\alpha )=ca\sin (180-\beta )\] more...