
|

 (2) Properties : (i) It is always a scalar which is positive if angle between the vectors is acute (i.e., < 90°) and negative if angle between them is obtuse (i.e. 90°<q < 180°).
(ii) It is commutative, i.e. \[\overrightarrow{A}\,.\,\overrightarrow{B}\,=\,\overrightarrow{B}\,.\,\overrightarrow{A}\]
(iii) It is distributive, i.e. \[\overrightarrow{A}\,.\,(\overrightarrow{B}+\overrightarrow{C})\,=\overrightarrow{A}\,.\,\overrightarrow{B}\,+\overrightarrow{A}\,.\,\overrightarrow{C}\]
(iv) As by definition \[\overrightarrow{A}\,.\,\overrightarrow{B}=AB\,\cos \theta \] more...
(2) Properties : (i) It is always a scalar which is positive if angle between the vectors is acute (i.e., < 90°) and negative if angle between them is obtuse (i.e. 90°<q < 180°).
(ii) It is commutative, i.e. \[\overrightarrow{A}\,.\,\overrightarrow{B}\,=\,\overrightarrow{B}\,.\,\overrightarrow{A}\]
(iii) It is distributive, i.e. \[\overrightarrow{A}\,.\,(\overrightarrow{B}+\overrightarrow{C})\,=\overrightarrow{A}\,.\,\overrightarrow{B}\,+\overrightarrow{A}\,.\,\overrightarrow{C}\]
(iv) As by definition \[\overrightarrow{A}\,.\,\overrightarrow{B}=AB\,\cos \theta \] more...
 If \[\overrightarrow{R}\] makes an angle a with x axis, b with y axis and \[\gamma \]with z axis, then
\[\Rightarrow q\] \[\cos \alpha =\frac{{{R}_{x}}}{R}=\frac{{{R}_{x}}}{\sqrt{R_{x}^{2}+R_{y}^{2}+R_{z}^{2}}}=l\]
\[\Rightarrow \] \[\cos \beta =\frac{{{R}_{y}}}{R}=\frac{{{R}_{y}}}{\sqrt{R_{x}^{2}+R_{y}^{2}+R_{z}^{2}}}=m\]
\[\Rightarrow \] \[\cos \gamma =\frac{{{R}_{z}}}{R}=\frac{{{R}_{z}}}{\sqrt{R_{x}^{2}+R_{y}^{2}+R_{z}^{2}}}=n\]
Where l, m, n are called Direction Cosines of the vector \[\overrightarrow{R}\] and \[{{l}^{2}}+{{m}^{2}}+{{n}^{2}}=\]\[{{\cos }^{2}}\alpha +{{\cos }^{2}}\beta +{{\cos }^{2}}\gamma =\frac{R_{x}^{2}+R_{y}^{2}+R_{z}^{2}}{R_{x}^{2}+R_{y}^{2}+R_{z}^{2}}=1\]
Note :
If \[\overrightarrow{R}\] makes an angle a with x axis, b with y axis and \[\gamma \]with z axis, then
\[\Rightarrow q\] \[\cos \alpha =\frac{{{R}_{x}}}{R}=\frac{{{R}_{x}}}{\sqrt{R_{x}^{2}+R_{y}^{2}+R_{z}^{2}}}=l\]
\[\Rightarrow \] \[\cos \beta =\frac{{{R}_{y}}}{R}=\frac{{{R}_{y}}}{\sqrt{R_{x}^{2}+R_{y}^{2}+R_{z}^{2}}}=m\]
\[\Rightarrow \] \[\cos \gamma =\frac{{{R}_{z}}}{R}=\frac{{{R}_{z}}}{\sqrt{R_{x}^{2}+R_{y}^{2}+R_{z}^{2}}}=n\]
Where l, m, n are called Direction Cosines of the vector \[\overrightarrow{R}\] and \[{{l}^{2}}+{{m}^{2}}+{{n}^{2}}=\]\[{{\cos }^{2}}\alpha +{{\cos }^{2}}\beta +{{\cos }^{2}}\gamma =\frac{R_{x}^{2}+R_{y}^{2}+R_{z}^{2}}{R_{x}^{2}+R_{y}^{2}+R_{z}^{2}}=1\]
Note :

 Now as for any vector \[\overrightarrow{A}=A\,\hat{n}\]
so, \[{{\overrightarrow{R}}_{x}}=\hat{i}{{R}_{x}}\] and \[{{\overrightarrow{R}}_{y}}=\hat{j}{{R}_{y}}\]
so \[\overrightarrow{R}=\hat{i}{{R}_{x}}+\hat{j}{{R}_{y}}\] ...(i)
But from figure \[{{R}_{x}}=R\cos \theta \] ...(ii)
and \[{{R}_{y}}=R\sin \theta \] ...(iii)
Since R and q are usually known, Equation (ii) and (iii) give the magnitude of the components of \[\overrightarrow{R}\] along x and y-axes respectively.
Here it is worthy to note once more...
Now as for any vector \[\overrightarrow{A}=A\,\hat{n}\]
so, \[{{\overrightarrow{R}}_{x}}=\hat{i}{{R}_{x}}\] and \[{{\overrightarrow{R}}_{y}}=\hat{j}{{R}_{y}}\]
so \[\overrightarrow{R}=\hat{i}{{R}_{x}}+\hat{j}{{R}_{y}}\] ...(i)
But from figure \[{{R}_{x}}=R\cos \theta \] ...(ii)
and \[{{R}_{y}}=R\sin \theta \] ...(iii)
Since R and q are usually known, Equation (ii) and (iii) give the magnitude of the components of \[\overrightarrow{R}\] along x and y-axes respectively.
Here it is worthy to note once more...
 \[\tan {{\alpha }_{1}}=\frac{B\sin \theta }{A+B\cos \theta }\]
and \[\tan {{\alpha }_{2}}=\frac{B\sin \,(180-\theta )}{A+B\cos \,(180-\theta )}\]
But \[\sin (180-\theta )=\sin \theta \] and \[\cos (180-\theta )=-\cos \theta \] \[\Rightarrow \]
\[\tan {{\alpha }_{2}}=\frac{B\sin \theta }{A-B\cos \theta }\]
\[\tan {{\alpha }_{1}}=\frac{B\sin \theta }{A+B\cos \theta }\]
and \[\tan {{\alpha }_{2}}=\frac{B\sin \,(180-\theta )}{A+B\cos \,(180-\theta )}\]
But \[\sin (180-\theta )=\sin \theta \] and \[\cos (180-\theta )=-\cos \theta \] \[\Rightarrow \]
\[\tan {{\alpha }_{2}}=\frac{B\sin \theta }{A-B\cos \theta }\]
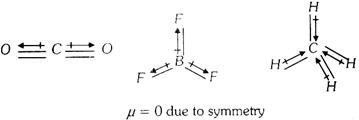 Unsymmetrical polyatomic molecules always have net value of dipole moment, thus such molecules are polar in more...
Unsymmetrical polyatomic molecules always have net value of dipole moment, thus such molecules are polar in more...
You need to login to perform this action.
You will be redirected in
3 sec
