Separating the Components of Mixtures
The different methods by which we can separate the components of mixtures are : Evaporation, Centrifugation, Decentation, Distillation, Fractional Distillation, Using separation funnel, Chromatography, and Sublimation. The method used is determined by the nature of mixtures. We will discuss each methods separately as below:

Evaporation
This methods is used to separate the dissolved material from the solvent. When we evaporate the solution, the solvent get evaporated and the solute is left behind. For example, we can separate the mixture of salt and water by this methods.

Centrifugation
In this method, when the mixture is spun rapidly, the denser particles are forced to settle at the bottom and lighter particles stays at
more...
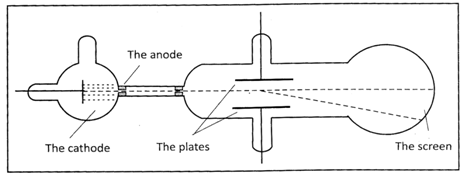 Through the gap, a small beam of cathode rays got out of the area of the cathode more...
Through the gap, a small beam of cathode rays got out of the area of the cathode more...
