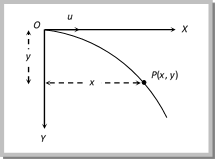
 The vertical displacement y is governed by \[y=\frac{1}{2}g{{t}^{2}}\] ?. (ii)
(since initial vertical velocity is zero)
By substituting the value of t in equation (ii) \[y=\frac{1}{2}\frac{g\,{{x}^{2}}}{{{u}^{2}}}\]
Sample problems based on trajectory
Problem 66. An aeroplane is flying at a constant horizontal velocity of 600 km/hr at an elevation of 6 km towards a point directly above the target on the earth?s surface. At an appropriate time, the pilot releases a ball so that it strikes the target at the earth. The ball will appear to be falling [MP PET 1993]
(a) On a parabolic path as seen by pilot in the plane
(b) Vertically along a straight path as seen by an observer on the ground near the target
(c) On a parabolic path as seen by an observer on the ground near the target
(d) On a zig-zag path as seen by pilot in the plane
Solution: (c)
The vertical displacement y is governed by \[y=\frac{1}{2}g{{t}^{2}}\] ?. (ii)
(since initial vertical velocity is zero)
By substituting the value of t in equation (ii) \[y=\frac{1}{2}\frac{g\,{{x}^{2}}}{{{u}^{2}}}\]
Sample problems based on trajectory
Problem 66. An aeroplane is flying at a constant horizontal velocity of 600 km/hr at an elevation of 6 km towards a point directly above the target on the earth?s surface. At an appropriate time, the pilot releases a ball so that it strikes the target at the earth. The ball will appear to be falling [MP PET 1993]
(a) On a parabolic path as seen by pilot in the plane
(b) Vertically along a straight path as seen by an observer on the ground near the target
(c) On a parabolic path as seen by an observer on the ground near the target
(d) On a zig-zag path as seen by pilot in the plane
Solution: (c)
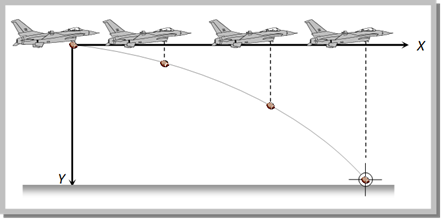 The path of the ball appears parabolic to a observer near the target because it is at rest. But to a Pilot the path appears straight line because the horizontal velocity of aeroplane and the ball are equal, so the relative horizontal displacement is zero.
Problem 67. The barrel of a gun and the target are at the same height. As soon as the gun is fired, the target is also released. In which of the following cases, the bullet will not strike the target
(a) Range of projectile is less than the initial distance between the gun and the target
(b) Range of projectile is more than the initial distance between the gun and the target
(c) Range of projectile is equal to the initial distance between the gun and target
(d) Bullet will always strike the target
Solution: (a) Condition for hitting of bullet with target initial distance between the gun and target \[\le \]Range of projectile.
Problem 68. A ball rolls off top of a staircase with a horizontal velocity u m/s. If the steps are h metre high and b mere wide, the ball will just hit the edge of nth more...
The path of the ball appears parabolic to a observer near the target because it is at rest. But to a Pilot the path appears straight line because the horizontal velocity of aeroplane and the ball are equal, so the relative horizontal displacement is zero.
Problem 67. The barrel of a gun and the target are at the same height. As soon as the gun is fired, the target is also released. In which of the following cases, the bullet will not strike the target
(a) Range of projectile is less than the initial distance between the gun and the target
(b) Range of projectile is more than the initial distance between the gun and the target
(c) Range of projectile is equal to the initial distance between the gun and target
(d) Bullet will always strike the target
Solution: (a) Condition for hitting of bullet with target initial distance between the gun and target \[\le \]Range of projectile.
Problem 68. A ball rolls off top of a staircase with a horizontal velocity u m/s. If the steps are h metre high and b mere wide, the ball will just hit the edge of nth more... | \[\sigma \]-Bond | \[\pi \]-Bond | |||||||||||
| Formed by End to End overlap of AO?s. | Formed by lateral overlap of \[p\]-orbitals. | |||||||||||
| Has cylindrical charge symmetry about bond axis. | Has maximum charge density in the cross-sectional plane of the orbitals. | |||||||||||
| Has free rotation | No free rotation, i.e., frozen rotation | |||||||||||
| more...
Dipole moment, resonance and reaction intermediates
Hybridisation in Organic Compounds
(1) Due to differences in electronegativity polarity developes between two adjacent atoms in the molecule (i.e., in a bond). The degree of polarity of a bond is called dipole moment. Dipole moment is represented by \[\mu \] and its unit is Debye (D).
\[\mu =e\times l\]
Where, \[e=\] magnitued of separated charge in e.s.u., \[l=\]internuclear distance between two atoms i.e., bond length in cm.
The dipole moment is denoted by arrow head pointing towards the positive to the negative end (?).
(2) Dipole moment of the compound does not depend only on the polarity of the bond but also depends on the shape of the molecule.Dipole moment of symmetrical compound is always zero, (\[\mu =0\]). Symmetrical compounds are those compounds which fulfil following two conditions,
(i) Central atom is bonded with the same atoms or groups.Examples:
\[\underset{\text{Symmetrical molecules}}{\mathop{{{H}_{2}},B{{F}_{3}},C{{S}_{2}},C{{H}_{2}}=C{{H}_{2}},CH\equiv CH}}\,\]
(ii) Central atom should have no lone pair of electrons.
Examples: \[\underset{\text{Symmetrical molecules}}{\mathop{CC{{l}_{4}},\,C{{H}_{4}},B{{H}_{3}},C{{O}_{2}}}}\,\] \[\underset{\text{Unsymmetrical molecules}}{\mathop{{{H}_{2}}\overset{.\,\,.}{\mathop{O}}\,,\,\,\,\,{{H}_{2}}\overset{.\,\,.}{\mathop{S}}\,}}\,\]
Note: q Compounds which have regular tetrahedral structure has no dipole moment.
(3) \[\mu \propto \]electronegativity of central atom or surrounding atoms present on the central atom of the molecule.
\[\underset{\begin{smallmatrix}
\text{Electronegativity in decreasing order} \\
\mu \text{ is also in decreasing order }
\end{smallmatrix}}{\mathop{\xrightarrow{CH{{F}_{3}} CHC{{l}_{3}} CHB{{r}_{3}} CH{{I}_{3}}}}}\,\]
\[\underset{\begin{smallmatrix}
\text{Electronegativity of central atom is in decreasing } \\
\text{order }\mu \text{ is also in decreasing order }
\end{smallmatrix}}{\mathop{\xrightarrow{\,\,\,\,N{{H}_{3}} P{{H}_{3}} As{{H}_{3}} Sb{{H}_{3}}\ \ \ \ }}}\,\]
Note: q Decreasing order of dipole moment in \[C{{H}_{3}}Cl,\,C{{H}_{2}}C{{l}_{2}},\,CHC{{l}_{3}}\] and \[CC{{l}_{4}}\] is
\[C{{H}_{3}}Cl>C{{H}_{2}}C{{l}_{2}}>CHC{{l}_{3}}>CC{{l}_{4}}\]
m = 1.86 D 1.62 D 1.03 0
q Alkynes has larger dipole moment because the electronegativity of \[sp-C\] is more than that of \[s{{p}^{2}}-C\].
(4) \[\mu \] cis \[>\,\mu \] trans in geometrical isomers.
(5) Dipole moment of the trans derivative of the compound \[(a)(b)C=C(a)(b)\] will only be zero if both \[a\] and \[b\] will be in the form of atoms.
Example:
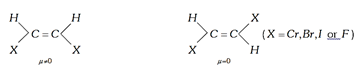 If both will not be atoms then \[\mu \] trans may or may not be zero.
If group have non-linear moments, then the dipole moment of the trans isomer will not be zero. If group have linear moments, then the dipole moment of the trans isomer will be zero.
Example:
If both will not be atoms then \[\mu \] trans may or may not be zero.
If group have non-linear moments, then the dipole moment of the trans isomer will not be zero. If group have linear moments, then the dipole moment of the trans isomer will be zero.
Example:  (6) Dipole moment of disubstituted benzene
(i) When both groups \[X\] and \[Y\] are electron donating or both groups are electron with drawing
(6) Dipole moment of disubstituted benzene
(i) When both groups \[X\] and \[Y\] are electron donating or both groups are electron with drawing
 Then, \[\mu =\sqrt{\mu _{1}^{2}+\mu _{2}^{2}+2{{\mu }_{1}}{{\mu }_{2}}\cos \theta }\]
Where, \[{{\mu }_{1}}=\] dipole moment of bond\[C-X\], \[{{\mu }_{2}}=\] dipole moment of bond \[C-Y\], \[\theta =\] angle between \[X\]and \[Y.\]
If value of \[\theta \] will be more, then \[\cos \theta \] will be less. Hence, dipole moment will be as,
\[\underset{\mu \text{ in decreasing order}}{\mathop{\xrightarrow{o-\text{derivative more...
Then, \[\mu =\sqrt{\mu _{1}^{2}+\mu _{2}^{2}+2{{\mu }_{1}}{{\mu }_{2}}\cos \theta }\]
Where, \[{{\mu }_{1}}=\] dipole moment of bond\[C-X\], \[{{\mu }_{2}}=\] dipole moment of bond \[C-Y\], \[\theta =\] angle between \[X\]and \[Y.\]
If value of \[\theta \] will be more, then \[\cos \theta \] will be less. Hence, dipole moment will be as,
\[\underset{\mu \text{ in decreasing order}}{\mathop{\xrightarrow{o-\text{derivative more...
Conductor and Conductance
Metallic and Electrolytic conductors.
(1) Conductors and Non – conductors: All substances do not conduct electrical current. The substances which allow the passage of electric current are called conductors. The best metal conductors are such as copper, silver, tin, etc. On the other hand, the substances which do not allow the passage of electric current through them are called non-conductors or insulators. Some common examples of insulators are rubber, wood, wax, etc.
(2) Types of conductors: The conductors are broadly classified into two types,
(i) Metallic conductors or electronic conductors.
(a) In metallic conductors, flow of electricity takes place without the decomposition of the substances.
(b) Flow of electricity is due to the flow of electrons only i.e., there is no flow of matter.
(c) In addition to metals, graphite and certain minerals also conduct electricity due to presence of free electrons in them, hence they are collectively called as electronic conductors.
(d) Metallic conduction decreases with increase of temperature. This is because kernels start vibrating which produce hinderance in the flow of electrons.
(e) The resistance offered by metals is also due to vibrating kernels.
(f) Metallic conductors obey Ohm's law.
(ii) Electrolytic conductors or Ionic conductors
(a) In electrolytic conductors flow of electricity takes place by the decomposition of the substance (Electrolyte).
(b) Flow of electricity is due to the movement of ions and hence there is flow of matter.
(c) Solutions of acids, bases and salts are the examples of electrolytic conductors.
(d) The electrolytic conduction will not occur unless the ions of the electrolyte are free to move. Therefore, these substances do not conduct electricity in the solid state but conduct electricity in the molten state or in their aqueous solutions.
(e) The electrical conduction increases with increase of temperature. This is generally due to increase in dissociation or decrease in the interionic attractions.
(f) The resistance shown by an electrolytic solution is due to factors like interionic attractions, viscosity of solvent etc.
(g) Electrolytic conductors also obey Ohm's law.
(h) All electrolytes do not ionise to the same extent in solution. On this basis, electrolytes are broadly divided into two types: strong electrolytes and weak electrolytes.
Strong electrolytes: The electrolytes which are almost completely dissociated into ions in solution are called strong electrolytes. For example, \[NaCl,KCl,HCl,NaOH,N{{H}_{4}}N{{O}_{3}},\]etc.
Weak electrolytes: The electrolytes which do not ionise completely in solution are called weak electrolytes. For example, \[C{{H}_{3}}COOH,{{H}_{2}}C{{O}_{3}},{{H}_{3}}B{{O}_{3}},HCN,HgC{{l}_{2}},ZnC{{l}_{2}},N{{H}_{4}}OH,\]etc. Thus in case of weak electrolytes, an equilibrium is established between the unionised electrolyte and the ions formed in solution. The extent of ionisation of a weak electrolyte is expressed in terms of degree of ionisation or degree of dissociation. It is defined as the fraction of total number of molecules of the electrolyte which ionise in the solution. It is generally denoted by alpha \[(\alpha ).\] For strong electrolytes, \[\alpha \] is almost equal to 1 and for weak electrolytes, it more...
Cell Constant and Electrochemical Cells
“Electrochemical cell or Galvanic cell is a device in which a spontaneous redox reaction is used to convert chemical energy into electrical energy i.e. electricity can be obtained with the help of oxidation and reduction reaction”.
(1) Characteristics of electrochemical cell: Following are the important characteristics of electrochemical cell,
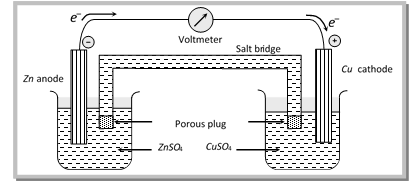
(i) Electrochemical cell consists of two vessels, two electrodes, two electrolytic solutions and a salt bridge.
(ii) The two electrodes taken are made of different materials and usually set up in two separate vessels.
(iii) The electrolytes are taken in the two different vessels called as half - cells.
(iv) The two vessels are connected by a salt bridge/porous pot.
(v) The electrode on which oxidation takes place is called the anode (or – ve pole) and the electrode on which reduction takes place is called the cathode (or + ve pole).
 (vi) In electrochemical cell, ions are discharged only on the cathode.
(vii) Like electrolytic cell, in electrochemical cell, from outside the electrolytes electrons flow from anode to cathode and current flow from cathode to anode.
(viii) For electrochemical cell,
\[{{E}_{cell}}=+ve,\,\,\,\Delta G=-ve.\]
(ix) In a electrochemical cell, cell reaction is exothermic.
(2) Salt bridge and its significance
(i) Salt bridge is U – shaped glass tube filled with a gelly like substance, agar – agar (plant gel) mixed with an electrolyte like KCl, KNO3, NH4NO3 etc.
(ii) The electrolytes of the two half-cells should be inert and should not react chemically with each other.
(iii) The cation as well as anion of the electrolyte should have same ionic mobility and almost same transport number, viz. \[KCl,\,KN{{O}_{3}},\,N{{H}_{4}}N{{O}_{3}}\]etc.
(iv) The following are the functions of the salt bridge,
(a) It connects the solutions of two half - cells and completes the cell circuit.
(b) It prevent transference or diffusion of the solutions from one half cell to the other.
(c) It keeps the solution of two half - cells electrically neutral.
(d) It prevents liquid – liquid junction potential i.e. the potential difference which arises between two solutions when they contact with each other.
Note : q Salt bridge can be replaced by a porous partition which allows the migration of ions without intermixing of solution.
q \[KCl\,(aq)\] cannot be used as a salt bridge for the cell, \[Cu(s)\left| CuS{{O}_{4}}(aq) \right|\,\left| AgN{{O}_{3}}(aq) \right|\,Ag(s)\].
Because \[AgCl\] is precipitated as follows, \[AgN{{O}_{3}}+KCl\xrightarrow{{}}\underset{(\text{ppt}\text{.})}{\mathop{AgCl}}\,\downarrow +KN{{O}_{3}}\]
(3) Representation of an electrochemical cell
(i) The interfaces across which a potential difference exists are shown by a semicolon (;) or a single vertical line (\[|\]). For example, the two half- cells of the following electrochemical cell can be represented as follows,
\[Zn(s)+C{{u}^{2+}}(aq)\xrightarrow{{}}Z{{n}^{2+}}(aq)+Cu(s);\] \[Zn\] ;\[Z{{n}^{2+}}\] or \[Zn|Z{{n}^{2+}}\] and \[C{{u}^{2+}}\];\[Cu\] or \[C{{u}^{2+}}|Cu\]
These indicate that potential difference exists at the \[Zn\] and \[Z{{n}^{2+}}\] ions interface, and similarly at the \[C{{u}^{2+}}\] and \[Cu\] more...
(vi) In electrochemical cell, ions are discharged only on the cathode.
(vii) Like electrolytic cell, in electrochemical cell, from outside the electrolytes electrons flow from anode to cathode and current flow from cathode to anode.
(viii) For electrochemical cell,
\[{{E}_{cell}}=+ve,\,\,\,\Delta G=-ve.\]
(ix) In a electrochemical cell, cell reaction is exothermic.
(2) Salt bridge and its significance
(i) Salt bridge is U – shaped glass tube filled with a gelly like substance, agar – agar (plant gel) mixed with an electrolyte like KCl, KNO3, NH4NO3 etc.
(ii) The electrolytes of the two half-cells should be inert and should not react chemically with each other.
(iii) The cation as well as anion of the electrolyte should have same ionic mobility and almost same transport number, viz. \[KCl,\,KN{{O}_{3}},\,N{{H}_{4}}N{{O}_{3}}\]etc.
(iv) The following are the functions of the salt bridge,
(a) It connects the solutions of two half - cells and completes the cell circuit.
(b) It prevent transference or diffusion of the solutions from one half cell to the other.
(c) It keeps the solution of two half - cells electrically neutral.
(d) It prevents liquid – liquid junction potential i.e. the potential difference which arises between two solutions when they contact with each other.
Note : q Salt bridge can be replaced by a porous partition which allows the migration of ions without intermixing of solution.
q \[KCl\,(aq)\] cannot be used as a salt bridge for the cell, \[Cu(s)\left| CuS{{O}_{4}}(aq) \right|\,\left| AgN{{O}_{3}}(aq) \right|\,Ag(s)\].
Because \[AgCl\] is precipitated as follows, \[AgN{{O}_{3}}+KCl\xrightarrow{{}}\underset{(\text{ppt}\text{.})}{\mathop{AgCl}}\,\downarrow +KN{{O}_{3}}\]
(3) Representation of an electrochemical cell
(i) The interfaces across which a potential difference exists are shown by a semicolon (;) or a single vertical line (\[|\]). For example, the two half- cells of the following electrochemical cell can be represented as follows,
\[Zn(s)+C{{u}^{2+}}(aq)\xrightarrow{{}}Z{{n}^{2+}}(aq)+Cu(s);\] \[Zn\] ;\[Z{{n}^{2+}}\] or \[Zn|Z{{n}^{2+}}\] and \[C{{u}^{2+}}\];\[Cu\] or \[C{{u}^{2+}}|Cu\]
These indicate that potential difference exists at the \[Zn\] and \[Z{{n}^{2+}}\] ions interface, and similarly at the \[C{{u}^{2+}}\] and \[Cu\] more...
Adsorption and Adsorption isotherm Adsorption. (1) Definition : The phenomenon of attracting and retaining the molecules of a substance on the surface of a liquid or solid resulting in to higher concentration of the molecules on the surface is called adsorption. (2) Causes of adsorption : Unbalanced forces of attraction or free valencies which is present at the solid or liquid surface, have the property to attract and retain the molecules of a gas or a dissolved substance on to their surfaces with which they come in contact.
 Example : (i) Ammonia gas placed in contact with charcoal gets adsorbed on the charcoal whereas ammonia gas placed in contact with water gets absorbed into water, giving \[N{{H}_{4}}OH\] solution of uniform concentration. (ii) If silica gel is placed in a vessel containing water vapours, the latter are adsorbed on the former. On the other hand, if anhydrous \[CaC{{l}_{2}}\] is kept in place of silica gel, absorption takes places as the water vapours are uniformly distributed in \[CaC{{l}_{2}}\] to form hydrated calcium chloride \[(CaC{{l}_{2}}.\ 2{{H}_{2}}O)\]. Some basic terms which are used in adsorption
Example : (i) Ammonia gas placed in contact with charcoal gets adsorbed on the charcoal whereas ammonia gas placed in contact with water gets absorbed into water, giving \[N{{H}_{4}}OH\] solution of uniform concentration. (ii) If silica gel is placed in a vessel containing water vapours, the latter are adsorbed on the former. On the other hand, if anhydrous \[CaC{{l}_{2}}\] is kept in place of silica gel, absorption takes places as the water vapours are uniformly distributed in \[CaC{{l}_{2}}\] to form hydrated calcium chloride \[(CaC{{l}_{2}}.\ 2{{H}_{2}}O)\]. Some basic terms which are used in adsorption
|