Chemical Stoichiometry
Calculation based on chemical equations is known as chemical stoichiometry. Stoichiometry can be broadly classified into two groups: (1) Gravimetric analysis (Stoichiometry-I), (2) Volumetric analysis (Stoichiometry-II)
(1) Gravimetric analysis (Stoichiometry-I) : With the help of chemical equation, we can calculate the weights of various substances reacting and weight of substances formed. For example,
\[MgC{{O}_{3}}\xrightarrow{{}}MgO+C{{O}_{2}}\uparrow \]
This equation implies :
(i) 1 mol of \[MgC{{O}_{3}}\] gives 1 mol of \[MgO\] and 1 mol of \[C{{O}_{2}}\].
(ii) 84 g of \[MgC{{O}_{3}}\] (Mol. wt. of \[MgC{{O}_{3}}\]) gives 40 g of \[MgO\] and 44 g of \[C{{O}_{2}}\].
Hence, chemical equation provide us information regarding :
(i) Molar ratio of reactants and products.
(ii) Mass ratio between reactants and products.
(iii) Volume ratio between gaseous reactant and products.
The calculation based upon chemical equation (Stoichiometry–I) are based upon three types namely :
(a) Mass-mass relationship (b) Mass-volume relationship (c) Volume-volume relationship
(2) Volumetric analysis (Stoichiometry-II) : It is a method which involves quantitative determination of the amount of any substance present in a solution through volume measurements. For the analysis a standard solution is required. (A solution which contains a known weight of the solute present in known volume of the solution is known as standard solution.)
To determine the strength of unknown solution with the help of known (standard) solution is known as titration. Different types of titrations are possible which are summerised as follows :
(i) Redox titrations : To determine the strength of oxidising agents or reducing agents by titration with the help of standard solution of reducing agents or oxidising agents.
Examples:
\[\begin{align}
& \underline{\begin{align}
& \underline{\begin{align}
& \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{{K}_{2}}C{{r}_{2}}{{O}_{7}}+4{{H}_{2}}S{{O}_{4}}\to {{K}_{2}}S{{O}_{4}}+C{{r}_{2}}{{(S{{O}_{4}})}_{3}}+4{{H}_{2}}O+3[O] \\
& \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,[2FeS{{O}_{4}}+{{H}_{2}}S{{O}_{4}}+O\to F{{e}_{2}}{{(S{{O}_{4}})}_{3}}+{{H}_{2}}O]\times 3\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, \\
\end{align}} \\
& \,\,6FeS{{O}_{4}}+{{K}_{2}}C{{r}_{2}}{{O}_{7}}+7{{H}_{2}}S{{O}_{4}}\to 3Fe{{(S{{O}_{4}})}_{3}}+{{K}_{2}}S{{O}_{4}}+C{{r}_{2}}{{(S{{O}_{4}})}_{3}}7{{H}_{2}}O \\
& \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,2KMn{{O}_{4}}+3{{H}_{2}}S{{O}_{4}}\to {{K}_{2}}S{{O}_{4}}+2MnS{{O}_{4}}+3{{H}_{2}}O+5[O] \\
& \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,[2FeS{{O}_{4}}+{{H}_{2}}S{{O}_{4}}+O\to F{{e}_{2}}{{(S{{O}_{4}})}_{3}}+{{H}_{2}}O]\times 5\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, \\
\end{align}} \\
& 10FeS{{O}_{4}}+2KMn{{O}_{4}}+8{{H}_{2}}S{{O}_{4}}\to 5F{{e}_{2}}{{(S{{O}_{4}})}_{3}}+{{K}_{2}}S{{O}_{4}}+2MnS{{O}_{4}}+8{{H}_{2}}O \\
\end{align}\]
Similarly with \[{{H}_{2}}{{C}_{2}}{{O}_{4}}\]
\[2KMn{{O}_{4}}+3{{H}_{2}}S{{O}_{4}}+5{{H}_{2}}{{C}_{2}}{{O}_{4}}\to {{K}_{2}}S{{O}_{4}}+2MnS{{O}_{4}}+8{{H}_{2}}O+10C{{O}_{2}}\] etc.
(ii) Acid-base titrations : To determine the strength of acid or base with the help of standard solution of base or acid.
Example: \[NaOH+HCl\to NaCl+{{H}_{2}}O\] and \[NaOH+C{{H}_{3}}COOH\to C{{H}_{3}}COONa+{{H}_{2}}O\] etc.
(iii) Iodiometric titrations : To determine the reducing agents with the help of standard iodine solution is known as iodiometry.
For example: \[\underset{\text{Reducing agent}}{\mathop{A{{s}_{2}}{{O}_{3}}}}\,+2{{I}_{2}}+2{{H}_{2}}O\to A{{s}_{2}}{{O}_{3}}+4HI\]
\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}+{{I}_{2}}\to N{{a}_{2}}{{S}_{4}}{{O}_{6}}+2NaI\]
(iv) Iodometric titrations : To determine the oxidising agent indirectly by titration of liberated \[{{I}_{2}}\] with the help of standard hypo solution is known as iodometric titrations.
Examples: Oxidising agents such as \[KMn{{O}_{4}},\,{{K}_{2}}C{{r}_{2}}{{O}_{7}},\,CuS{{O}_{4}}\], ferric salts, etc. are reduced quantitatively when treated with large excess of KI in acidic or neutral medium and liberate equivalent amount of \[{{I}_{2}}\].
\[2CuS{{O}_{4}}+4KI\to C{{u}_{2}}{{I}_{2}}+2{{K}_{2}}S{{O}_{4}}+{{I}_{2}}\]
\[K{{r}_{2}}C{{r}_{2}}{{O}_{7}}+7{{H}_{2}}S{{O}_{4}}+6KI\to C{{r}_{2}}{{(S{{O}_{4}})}_{3}}+4{{K}_{2}}S{{O}_{4}}+7{{H}_{2}}O+3{{I}_{2}}\]
This \[{{I}_{2}}\] is estimated with hypo
\[{{I}_{2}}+2N{{a}_{2}}{{S}_{2}}{{O}_{3}}\to N{{a}_{2}}{{S}_{4}}{{O}_{6}}+2NaI\]
(v) Precipitation titrations : To determine the anions like \[C{{N}^{-}},\ AsO_{3}^{3-},\ PO_{4}^{3-},\ {{X}^{-}}\] etc, by precipitating with \[AgN{{O}_{3}}\] provides examples of
more...
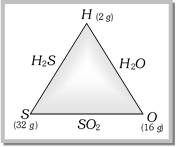 Example : Formation of \[{{H}_{2}}S,\,\,{{H}_{2}}O\] and \[S{{O}_{2}}\] can be done as follows,
(i) Hydrogen combines with sulphur forming hydrogen sulphide; 2gm. of hydrogen reacts with 32gm of sulphur. (ii) Hydrogen combines oxygen forming water; 2 gm. of hydrogen reacts with 16 gm. of oxygen. (iii) Sulphur combines with more...
Example : Formation of \[{{H}_{2}}S,\,\,{{H}_{2}}O\] and \[S{{O}_{2}}\] can be done as follows,
(i) Hydrogen combines with sulphur forming hydrogen sulphide; 2gm. of hydrogen reacts with 32gm of sulphur. (ii) Hydrogen combines oxygen forming water; 2 gm. of hydrogen reacts with 16 gm. of oxygen. (iii) Sulphur combines with more... 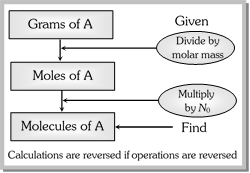 1 mole of a substance = \[6.022\times {{10}^{23}}\] species
The molar mass of a substance is the mass in grams of 1 mole of that substance.
\[\text{Mole of a substance }=\frac{\text{mass in grams}}{\text{molar mass}}\]
Under STP conditions when temperature is 273K and pressure is 1 atm, volume of one mole of an ideal gas is 22.4L
1 mole of a substance = \[6.022\times {{10}^{23}}\] species
The molar mass of a substance is the mass in grams of 1 mole of that substance.
\[\text{Mole of a substance }=\frac{\text{mass in grams}}{\text{molar mass}}\]
Under STP conditions when temperature is 273K and pressure is 1 atm, volume of one mole of an ideal gas is 22.4L