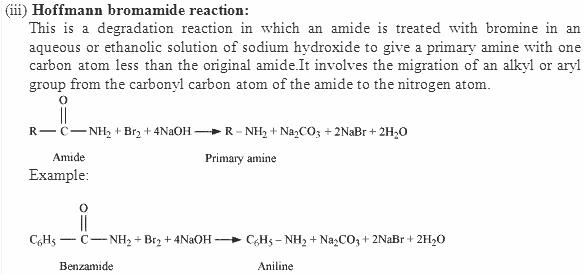
The Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one fewer carbon atom.
By this method the amide (–CONH2) group is converted into primary amino (– NH2) group.
R - CO - NH2 + Br2 + 4KOH → R - NH2 + 2KBR + K2CO3 + 2H2O
Amide Pri-amine
This is the most convenient method for preparing primary amines.
This method gives an amine containing one carbon atom less than amide.
The reaction is named after its discoverer: August Wilhelm von Hofmann. This reaction is also sometimes called the Hofmann degradation or the Harmon Process, and should not be confused with the Hofmann elimination.
The reaction of bromine with sodium hydroxide forms sodium hypobromite in situ, which transforms the primary amide into an intermediate isocyanate. The intermediate isocyanate is hydrolyzed to a primary amine, giving off carbon dioxide.
Hoffmann Bromamide reaction is being explained to you by Mr. Lalit Sardana(IIT-JEE AIR 243). He is an IIT JEE trainer and the Head of Sardana Tutorials. This video is helpful for the students appearing for 11th CBSE, 12th CBSE, JEE Mains, JEE Advanced, KCET, VITEEE, J and K Engineering exams.