| A 1 | B 2 | C 3 | D 4 | E 5 | F 6 | G 7 | H 8 | I 9 | more...
Cubes and Dices
Concept of Cubes
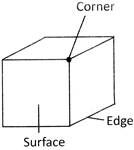
A cube is a three-dimensional figure which has 8 corners, 6 surfaces and 12 edges.
Type I
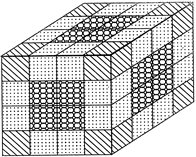
If a cube is painted on all of its surfaces with a colour and then divided into smaller cubes of equal size, then after separation, number of smaller cubes so obtained will be calculated as under:
No. of smaller cubes with three surfaces painted = 8
No. of smaller cubes with one surface painted \[= \left( n - 2 \right)~12\]
No. of smaller cubes with no surface painted \[={{\left( n - 2 \right)}^{2}}~6\]
No. of smaller cubes with no surface painted \[= {{\left( n - 2 \right)}^{3}}\]
Where, n = No. of divisions on the surface of the bigger cube
\[=\frac{length\,of\,edge\,of\,big\,cube}{length\,of\,edge\,of\,one\,smaller\,cube}\]
   Type II
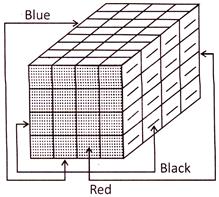
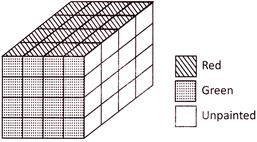
If a cube is painted on all of its surfaces with different colours and then divided into various smaller cubes of equal size.
Type II
If a cube is painted on all of its surfaces with different colours and then divided into various smaller cubes of equal size.
Nutrition
Nutrition in Plants
All living things need food to survive. Food gives energy to grow, reproduce, move and to work.
Nutrition is the process by which an organism obtains its food and utilize them. The nutrition can be categorised mainly into two types namely autotrophic and heterotrophic nutrition.
Autotrophic Nutrition
Green plants, algae and some bacteria can produce their own food by the process of photosynthesis. The process of photosynthesis occurs only when plants or algae or some bacteria have green pigment, called chlorophyll in their cells. In the process of photosynthesis, the leaves of plants convert water and carbon dioxide into glucose or sugar and oxygen with the help of energy from the sun. Plants take in carbon dioxide from air and water from the soil.
Heterotrophic Nutrition
The plants which do not contain chlorophyll obtain their food by heterotrophic mode of nutrition. There are mainly three types of plants which obtain their food by heterotrophic nutrition. These plants are:
Saprophytic plants
Saprophytes are the non-green plants that feed on dead and decaying organic matters derived from plants and animals. These organisms break down the organic matters by secreting digestive juices into it. For example, mushrooms and toadstool, etc.
 Parasitic Plants
Some non-green plants live inside or on other organisms and derive nutrition from them. Such plants are called parasites and the organisms on which the parasite lives are called hosts. For example, dodder is a parasitic plant that winds its yellowish and threadlike stems around other plants and draw nutrition from them.
Parasitic Plants
Some non-green plants live inside or on other organisms and derive nutrition from them. Such plants are called parasites and the organisms on which the parasite lives are called hosts. For example, dodder is a parasitic plant that winds its yellowish and threadlike stems around other plants and draw nutrition from them.
 Insectivorous plants
Some plants obtain a part of their food from insects. For example pitcher plants trap the insects in their modified leaf, kill them and digest them to obtain nutrients. In fact insectivorous plants grow in the soil which is not very rich in nutrients. So they get essential nutrients by eating insects.
Insectivorous plants
Some plants obtain a part of their food from insects. For example pitcher plants trap the insects in their modified leaf, kill them and digest them to obtain nutrients. In fact insectivorous plants grow in the soil which is not very rich in nutrients. So they get essential nutrients by eating insects.
 Nutrition in Animals
Animals cannot make their own food because they do not have green pigment named chlorophyll. Animals depend on plants or other animals for their food.
Nutrition in Animals involve Five Steps
Ingestion: Intake of food inside the body is called ingestion.
Digestion: Breaking down of large food molecules to smaller one is called digestion.
Absorption: The digested food is absorbed into blood stream through intestinal wall. This process is known as absorption.
Assimilation: The process of utilizing absorbed food by body cells for various metabolic processes is called assimilation.
Egestion: The process of removing undigested food out of body is called egestion.
Nutrition in Amoeba
Amoeba is a unicellular organism living in pond water. Amoeba has finger like projections called pseudopodia. Pseudopodia are false feet which is used by amoeba for catching and engulfing tiny food particles. The food is stored and digested into food vacuoles. more...
Nutrition in Animals
Animals cannot make their own food because they do not have green pigment named chlorophyll. Animals depend on plants or other animals for their food.
Nutrition in Animals involve Five Steps
Ingestion: Intake of food inside the body is called ingestion.
Digestion: Breaking down of large food molecules to smaller one is called digestion.
Absorption: The digested food is absorbed into blood stream through intestinal wall. This process is known as absorption.
Assimilation: The process of utilizing absorbed food by body cells for various metabolic processes is called assimilation.
Egestion: The process of removing undigested food out of body is called egestion.
Nutrition in Amoeba
Amoeba is a unicellular organism living in pond water. Amoeba has finger like projections called pseudopodia. Pseudopodia are false feet which is used by amoeba for catching and engulfing tiny food particles. The food is stored and digested into food vacuoles. more...
Fibre to Fabric
Fibre
Fibre is a natural or synthetic substance which is used to manufacture the other things.
Natural Fibre
Natural fibre is obtained from plants and animals. Fibres such as cotton, flax and jute are derived from plants. Fibres such as silk and wool are derived from animals.
Plant Fibres
The fibres which we get from plants are called plant fibres. Cotton, flax and jute are plant fibres.
Cotton: Cotton fibre is obtained from the cotton bolls of the cotton plant.
Flax: Flax fibre is soft, lustrous and flexible. It is stronger than cotton fibre but is less elastic. The finer grade of flax fibre is used for producing linen fabrics such as damasks, lace and sheeting. The coarser grades of flax fibre are used for manufacturing twine and rope. Flax fibre also forms the raw material for high quality paper industry.
Jute: Jute is a long, rough and shiny fibre. It is spun into coarse and strong threads. Jute is the cheapest natural fibre. It is composed of plant materials such as cellulose, lignin and pectin.
Animal Fibres
The fibres which we get from animals are called animal fibres. Wool and silk are animal fibres.
Silk: Silk is derived from cocoons of silkworm. Silkworms are reared for obtaining silk. This is known as sericulture. The various silk fabrics are crepe, satin, damask, taffeta, etc. Clothes made from silk fibre are soft, lustrous and comfortable to wear.
Wool: Wool is obtained by cutting the fleece from the body of some hairy animals such as sheep, goat, yak and camel. The main source of wool is sheep. Wool is used for making warm clothes.
Man Made Fibres
Man made fibres can be divided into two types namely regenerated fibres and synthetic fibres. Rayon and acetate are two regenerated fibres. Nylon, polyester, acrylic, etc. are synthetic fibres.
Regenerated Fibres
The regenerated fibres are made from natural materials and the material is processed to form the fibre structure. Regenerated fibres are derived from the cellulose found in cotton and wood pulp.
Acetate: Acetate is a weak fibre, but fibres of different diameters can be produced. Acetate fibre produces luxurious fabrics that look similar to silk. The fabric made from this fibre is wrinkle free, pliable and soft with a good drape.
Rayon: Rayon is strong and extremely absorbent. Rayon fibre does not melt but burns at high temperature. Fabric made from rayon wrinkle easily and may stretch when wet and shrink when washed.
Synthetic Fibres
Synthetic fibres are completely made from chemicals. These fibres are stronger than natural or regenerated fibres. Both synthetic and regenerated acetate fibres are thermoplastic, which means they are softened by heat. Synthetic fibres melt at very high temperature.
Acrylic Fibre: Acrylic fibre or acrylonitrile is made from natural gas and petroleum. The fabric made from acrylic fibre is soft and luxurious. Acrylic fibre is very sensitive more...
Physical and Chemical Changes
All the substances have certain properties such as state (solid, liquid or gas), size, color, smell, temperature, shape, composition and structure etc.
When properties of a substance become different, we say that a change has taken place in it. Changes are taking place all around us. Some changes are beneficial to us and some are harmful to us. As for example ripening of fruits is a beneficial change. On the other hand rusting of iron is a harmful change.
Type of Changes
Acids, Bases and Salts
Acids
Acids are present in things that we eat such as lemon and orange contains citric acid, tomato contains oxalic acid, vinegar contains acetic acid, yoghurt contains lactic acid and frizzy drinks contain carbonic acid. Acids found in foods are mild. Acids such as hydrochloric acid (HCI), sulphuric acid \[({{H}_{2}}S{{O}_{4}})\]and nitric acid \[(HN{{O}_{3}})\]are called mineral acids. They are strong acids. Touching strong acids can cause acid burns.
Acids are formed by dissolving oxides of non-metals such as carbon, sulphur and nitrogen in water. The oxides of non-metals are acidic because when dissolved in water they form acids. For example:
\[C{{O}_{2}}+{{H}_{2}}O\xrightarrow{{}}{{H}_{2}}C{{O}_{3}}\]
\[S{{O}_{3}}+{{H}_{2}}O\xrightarrow{{}}{{H}_{2}}S{{O}_{4}}\]
\[3N{{O}_{2}}+{{H}_{2}}O\xrightarrow[{}]{}NO+2HN{{O}_{3}}\]
Characteristics of Acidic Substances
The acidic substances show the following behaviour:
Weather, Climate and Adaptations of Animals to Climate
Weather
Atmospheric conditions include temperature, rainfall and the speed of the wind. Weather indicates the day to day atmospheric conditions. In addition to this, weather report for a place indicates humidity levels, time of sun rise and sun set.
Temperature
Temperature is a physical quantity expressing hot or cold. The maximum and minimum atmospheric temperature can be measured with the help of thermometer.
Humidity
Humidity is the amount of water vapour present in the air. The capacity of the air to hold water vapour increases with temperature.
The amount of water vapour present in the air expressed as a percentage of the amount of water needed for saturation at the same temperature is called relative humidity.
The relative humidity is measured with an instrument called hygrometer.
Rainfall, Snow and Fog
The humidity in air determines the chances of rainfall. Air when becomes saturated and temperature of atmosphere falls, the water vapour in the air condenses. When condensation takes place high above the ground, clouds are formed. When the droplets join together and become heavy, they come down as rainfall. If raindrops pass through a very cold layer of air, they freeze and come down as hailstone. The rainfall is measured in millimeters with an instrument called rain gauge.
Snow is formed when the temperature in the atmosphere is very low and the droplets in a cloud freeze into crystals of ice.
In winter after sunset, the water vapour near ground condenses due to low temperature. The condensed droplets in air are called fog.
Climate
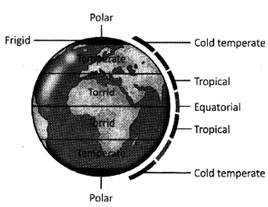
The average weather condition of a place for the long time is the climate of that place. The climate of a place is determined by its latitude. The five main latitude regions of earth's surface comprise geographical zones divided by major circles of latitude. The area around the equator, which is heated by the direct rays of sun, is hot or torrid zone. The area around the North Pole and the South Pole, which is cold because the rays of the sun slant the most, is called frigid zones. The region between the torrid and frigid zone is temperate zone.
Rainfall is another important factor that determines the climate of a region. The degree of rainfall depends on factors such as winds, closeness to the sea and the presence of the mountains.
 Adaptation of Animals to Climate
Animals have to adapt to the environment in which they live to cope up with the conditions like temperature, light, moisture and availability of food.
Cold Climate
Animals living in the Polar regions such as polar bears, ermines and penguins have thick coat of fur to conserve heat. The polar animals such as polar bears and seals have very thick layer of fat under their skin. This fat layer protects these animals from cold and acts as a store of more...
Adaptation of Animals to Climate
Animals have to adapt to the environment in which they live to cope up with the conditions like temperature, light, moisture and availability of food.
Cold Climate
Animals living in the Polar regions such as polar bears, ermines and penguins have thick coat of fur to conserve heat. The polar animals such as polar bears and seals have very thick layer of fat under their skin. This fat layer protects these animals from cold and acts as a store of more...
Wind, Storms and Cyclones
Wind
Moving air is called wind. Wind occurs due to the heating caused by the sun. The unequal heating of different parts of the earth causes the wind to blow. When the wind blows with slow speed it is called breeze. When the wind blows with high speed it is called storm.
Air Pressure
Air exerts pressure. Like temperature and humidity, air pressure also determines the weather of a place on a particular day. Air pressure can be measured with an instrument called barometer.
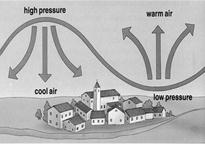 Wind is Produced Due to Uneven Heating on the Earth by the Sun
Uneven heating on the earth can take place by two situations:
Wind is Produced Due to Uneven Heating on the Earth by the Sun
Uneven heating on the earth can take place by two situations:
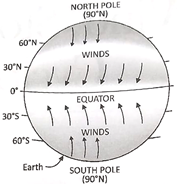
 Cyclones that develop over western Atlantic and eastern Pacific Ocean are called hurricanes. Cyclones that develop over western Pacific are called typhoons. Cyclones that develop over Indian Ocean, Bay of Bengal and Arabian Sea more...
Cyclones that develop over western Atlantic and eastern Pacific Ocean are called hurricanes. Cyclones that develop over western Pacific are called typhoons. Cyclones that develop over Indian Ocean, Bay of Bengal and Arabian Sea more...
Life Processes
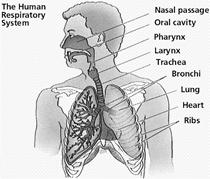
Respiration
Food provides energy to all living organisms. Food is first broken down into simple and soluble molecules. The simple molecules are then carried to all the cells. In cells, these molecules combine with oxygen to produce energy. This process of combination of simple food molecules with oxygen to produce energy is called respiration.
Types of Respiration
There are two types of respirations: aerobic respiration and anaerobic respiration.
 more...
more...
Motion and Time
Motion
If a body changes its position with time it is said to be in motion.
Different Types of Motion
Motion has been broadly classified into two groups. They are uniform motion, non-uniform motion.
Uniform Motion: If a body travels equal distance in equal interval of time then it is said to be uniform motion. For example, a car travels first 30 kilometres in one hour and next 30 kilometres again in one hour and so on then we say that car has uniform motion.
Non-Uniform Motion: If a body travels unequal distance in equal interval of time then it is called non-uniform motion. For example, a car travels first 30 kilometres in one hour and next 30 kilometres in half an hour then we say that, the car has non-uniform motion.
Speed
The speed of a moving body is the distance travelled per unit time. The speed of a moving body can be expressed as:
Distance = Speed \[\times \]Time
The SI unit of distance is metre (m) and of time is second (s), and the unit of speed is metre/second (m/s).
Time
It is important to measure time. If the time is not measured, one would not know small things such as when a train or plane will arrive. The unit of measurement of time is the time interval between two events. An event that occurs at regular interval of time is called periodic. For example, the rising of sun, changing of seasons, the waxing and waning of the moon are periodic. The rising of sun is used to measure day and cycle of seasons are used to mark a year.
Time Measuring Instruments
In ancient times, instruments such as sundial, hourglass and candle clock were used for measuring time.
Sundial
Sundial consists of a triangular metal plate fixed vertically at the centre of a circular plate. To measure time, sundial was placed in the sun with the triangular plate known as gnomon along the north-south direction. The position of the shadows formed by the gnomon at different times of day were used to mark the time on the circular plate or dial. Sundial told quite accurate time, but the limitation was that it could neither be used after sunset nor carried around.
Hourglass
Another instrument for measuring time was hourglass. Hourglass consisted of two glass bulbs connected through small opening. One of the glass bulb was filled with sand. Sand passed from one bulb to another through small opening. When all the sand from one bulb had passed to another, the instrument can be inverted to measure the time again.
Candle Clock
Another instrument for measuring time was candle clock. The candle was marked with numbers. As the candle burnt, the marking indicated time.
Pendulum Clock
The modern time measuring instrument is pendulum clock. An Italian astronomer Galileo Galilei discovered that the motion of a pendulum is periodic more...
Current Affairs CategoriesArchive
Trending Current Affairs
You need to login to perform this action. |