Modern Periodic Classification

Periodic Law
'Properties of elements are a periodic function of their atomic number Atomic number gives us the number of protons in the nucleus of an atom and this number increases by one in going from one element to the next. Elements, when arranged in order of increasing atomic number Z, lead us to the classification known as the Modern Periodic Table. Prediction of properties of elements could be made with more precision when elements were arranged on the basis of increasing atomic number.
Position of Elements in the Modern Periodic Table:
The Modern Periodic Table has 18 vertical columns known as 'groups' and 7 horizontal rows known as 'periods'. Let us see what decides the placing of an element in a certain group and period. All elements of a group contain same number of valence electrons, which justifies similar chemical properties. The atomic radius decreases in moving from left to right along a period. This is due to an increase in nuclear charge, which tends to pull the electrons closer to the nucleus and reduces the size of the atom. Atoms of different elements with the same number of occupied shells are placed in the same period. Na,
Mg, Al, Si, P, S, Cl and Ar belong to the third period of the Modern Periodic
Table, since the electrons in the atoms of these elements are filled in K, L and M shells.
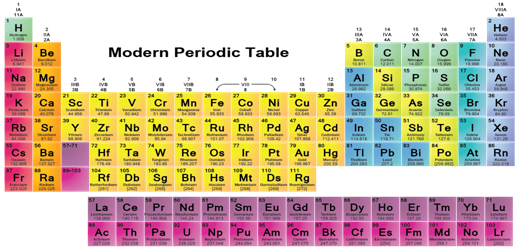

Metallic & Non-metallic Properties
Metals like Na and Mg are towards the left-hand side of the Periodic Table while the non-metals like sulphur and chlorine are found on the right-hand side. In the middle, we have silicon, which is classified as a semi-metal or metalloid because it exhibits some properties of both metals and non-metals. In the Modern Periodic Table, a zig-zag line separates metals from non-metals. The borderline elements - boron, silicon, germanium, arsenic, antimony, tellurium and polonium - are intermediate in properties and are called metalloids or semi-metals. Metals tend to lose electrons while forming bonds, that is, they are electropositive in nature.
As the effective nuclear charge acting on the valence shell electrons increases across a period, the tendency to lose electrons decreases. Down the group, the effective nuclear charge experienced by valence electrons is decreasing because the outermost electrons are farther away from the nucleus. Therefore, these can be lost easily. Hence metallic character decreases across a period
and increases down a group.
As the trends in the electro negativity show, non-metals are found on the right- hand side of the Periodic Table towards the top. These trends also help us to predict the nature of oxides formed by the elements.
It is know that the oxides
more...
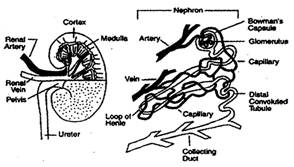
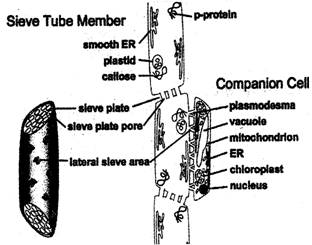
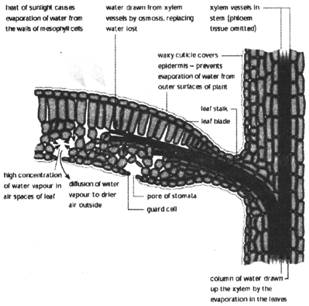 The other vascular tissue, phloem transports the prepared food material front leaves to the other parts of the plant. The phloem tissue consists of tsieve tube, companion cells and phloem parenchyma.
The other vascular tissue, phloem transports the prepared food material front leaves to the other parts of the plant. The phloem tissue consists of tsieve tube, companion cells and phloem parenchyma.
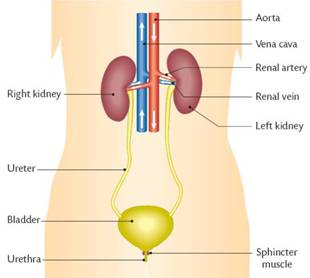
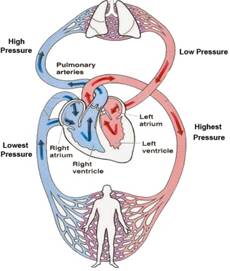 Human circulatory system, consists of heart and blood capillaries. The human heart is divided into four parts, called chambers. The four chambers of heart are left and right auricles and left and right ventricles. It is divided into two parts, by a thick layer of membrane, called septum. The left and right auricles and ventricles are connected with each other by a small opening called valves. The oxygenated blood from lungs enters into the heart through left auricles via pulmonary veins. When the left auricles contracts the blood is transferred to the left ventricles which expands. When the ventricles contracts the blood is pumped into the aorta for circulation to different parts of the body. The back flow of the blood is prevented by the closing of valves. After circulation through the body, the deoxygenated blood returns to the heart via vena cava into right auricles. When the auricles contracted the blood is forced into right ventricles through small opening called valves. This impure blood from right more...
Human circulatory system, consists of heart and blood capillaries. The human heart is divided into four parts, called chambers. The four chambers of heart are left and right auricles and left and right ventricles. It is divided into two parts, by a thick layer of membrane, called septum. The left and right auricles and ventricles are connected with each other by a small opening called valves. The oxygenated blood from lungs enters into the heart through left auricles via pulmonary veins. When the left auricles contracts the blood is transferred to the left ventricles which expands. When the ventricles contracts the blood is pumped into the aorta for circulation to different parts of the body. The back flow of the blood is prevented by the closing of valves. After circulation through the body, the deoxygenated blood returns to the heart via vena cava into right auricles. When the auricles contracted the blood is forced into right ventricles through small opening called valves. This impure blood from right more... 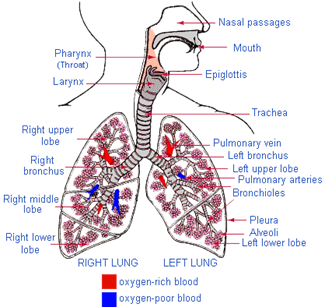 Air enters into our body through nostril. This air is filtered by fine hairs that is present in the inner lining of nostril. From there air passes through the wind pipe into lungs via trachea.
The trachea consists of rings of cartilage muscles, which prevents it from collapsing when we inhale the air. The trachea is further divided into two parts before entering into two lungs called bronchi. The bronchi when enters into the lungs, it gets further subdivided into smaller capillaries called bronchioles, which finally terminates in balloon like structure called alveoli.
The alveoli helps in the exchange of gases between lungs and blood vessels.
The spherical surface of alveoli increases the surface for the exchange of gases.
The blood brings carbon dioxide from the rest of the body and release it into lungs and takes up oxygen from lungs and carries it back to the body.
Air enters into our body through nostril. This air is filtered by fine hairs that is present in the inner lining of nostril. From there air passes through the wind pipe into lungs via trachea.
The trachea consists of rings of cartilage muscles, which prevents it from collapsing when we inhale the air. The trachea is further divided into two parts before entering into two lungs called bronchi. The bronchi when enters into the lungs, it gets further subdivided into smaller capillaries called bronchioles, which finally terminates in balloon like structure called alveoli.
The alveoli helps in the exchange of gases between lungs and blood vessels.
The spherical surface of alveoli increases the surface for the exchange of gases.
The blood brings carbon dioxide from the rest of the body and release it into lungs and takes up oxygen from lungs and carries it back to the body. 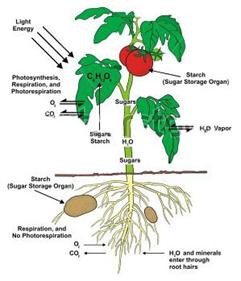 The stroma contains stacks (grana) of thylakoids, which are the site of photosynthesis. The thylakoids are flattened disks, bounded by a membrane with a lumen or thylakoid space within it. The site of photosynthesis is the thylakoid membrane, which contains integral and peripheral membrane protein complexes, including the pigments that absorb light energy, which form the more...
The stroma contains stacks (grana) of thylakoids, which are the site of photosynthesis. The thylakoids are flattened disks, bounded by a membrane with a lumen or thylakoid space within it. The site of photosynthesis is the thylakoid membrane, which contains integral and peripheral membrane protein complexes, including the pigments that absorb light energy, which form the more... 