Covalent bond was first proposed by Lewis in 1916. The bond formed between the two atoms by mutual sharing of electrons so as to complete their octets or duplets (in case of elements having only one shell) is called covalent bond or covalent linkage. A covalent bond between two similar atoms is non-polar covalent bond while it is polar between two different atom having different electronegativities. Covalent bond may be single, double or a triple bond. We explain covalent bond formation by Lewis octet rule.
Chlorine atom has seven electrons in the valency shell. In the formation of chlorine molecule, each chlorine atom contributes one electron and the pair of electrons is shared between two atoms. both the atoms acquire stable configuration of argon.
\[\underset{(2,\,8,\,7)}{\mathop{_{\bullet }^{\bullet }\underset{\bullet \,\,\bullet }{\overset{\bullet \,\,\bullet }{\mathop{Cl}}}\,\,\bullet }}\,\,\underset{(2,\,8,\,7)}{\mathop{\,\,\,*\underset{*\,\,*}{\overset{*\,\,*}{\mathop{Cl\,_{*}^{*}}}}\,}}\,\,\,\,\,\,\to \,\,\,\,\underset{\,(2,\,8,\,8)\,\,\,\,\,\,\,(2,\,8,8)}{\mathop{\,\underset{\bullet \,\,\bullet \,\,}{\overset{\bullet \,\,\bullet \,\,}{\mathop{_{\bullet }^{\bullet }Cl\,\,_{\,*}^{\,\bullet }}}}\,\,\,\underset{*\,\,*\,\,\,\,\,}{\overset{*\,\,*\,\,\,\,\,}{\mathop{Cl\,_{*}^{*}}}}\,}}\,\] or \[Cl-Cl\]
Some other examples are : \[{{H}_{2}}S,N{{H}_{3}},HCN,PC{{l}_{3}},P{{H}_{3,}}\] \[{{C}_{2}}{{H}_{2}},{{H}_{2}},{{C}_{2}}{{H}_{4}},SnC{{l}_{4}},FeC{{l}_{3}},B{{H}_{3}},\]graphite, \[BeC{{l}_{2}}\]etc.
(1) Conditions for formation of covalent bond
(i) The combining atoms should be short by 1, 2 or 3 electrons in the valency shell in comparison to stable noble gas configuration.
(ii) Electronegativity difference between the two atoms should be zero or very small.
(iii) The approach of the atoms towards one another should be accompanied by decrease of energy.
(2) Characteristics of covalent compounds
(i) These exist as gases or liquids under the normal conditions of temperature and pressure. Some covalent compounds exist as soft solids.
(ii) Diamond, Carborandum (SiC), Silica (SiO2), AlN etc. have giant three dimensional network structures; therefore have exceptionally high melting points otherwise these compounds have relatively low melting and boiling points.
(iii) In general covalent substances are bad conductor of electricity. Polar covalent compounds like HCl in solution conduct electricity. Graphite can conduct electricity in solid state since electrons can pass from one layer to the other.
(iv) These compounds are generally insoluble in polar solvent like water but soluble in non-polar solvents like benzene etc. some covalent compounds like alcohol, dissolve in water due to hydrogen bonding.
(v) The covalent bond is rigid and directional. These compounds, thus show isomerism (structural and space).
(vi) Covalent substances show molecular reactions. The reaction rates are usually low.
(vii) The number of electrons contributed by an atom of the element for sharing with other atoms is called covalency of the element. Covalency = 8 – [Number of the group to which element belongs]. The variable covalency of an element is equal to the total number of unpaired electrons in s, p and d-orbitals of its valency shell.
The element such as P, S, Cl, Br, I have vacant d-orbitals in their valency shell. These elements show variable covalency by increasing the number of unpaired electrons under excited conditions. The electrons from paired orbitals get excited to vacant d-orbitals of the same shell.
Four elements, H, N, O and F do not possess d-orbitals in their valency shell. Thus, such an excitation is not possible and variable valency is not shown by these elements. This is reason
more... 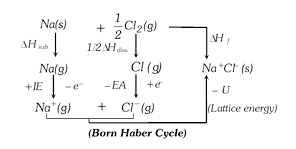 According to Hess's law of constant heat summation, heat of formation of an ionic solid is net resultant of the above changes. \[\Delta {{H}_{f}}=\Delta {{H}_{\text{Subl}\text{.}}}+\frac{1}{2}\Delta {{H}_{\text{diss}\text{.}}}+IE-EA-U\]
(2) Characteristics of electrovalent compounds
(i) Electrovalent compounds are generally crystalline is nature. The constituent ions are arranged in a regular way in their lattice.
(ii) Electrovalent compounds possess high melting and boiling points. Order of melting and boiling points in halides of sodium and oxides of IInd group elements is as,
\[NaF>NaCl>NaBr>NaI,\]\[MgO>CaO>BaO\]
(iii) Electrovalent compounds are hard and brittle in nature.
(iv) Electrovalent solids do not conduct electricity. While electrovalent compounds in the molten state or in solution conduct electricity.
(v) Electrovalent compounds are fairly soluble in polar solvents and insoluble in non-polar solvents.
(vi) The electrovalent bonds are non-rigid and non-directional. Thus these compound do not show space isomerism e.g. geometrical or optical isomerism.
(vii) Electrovalent compounds furnish ions in solution. The chemical reaction of these compounds are known as ionic reactions, which are fast.
\[{{K}^{+}}C{{l}^{-}}+\overset{+}{\mathop{Ag}}\,\overset{-}{\mathop{N{{O}_{3}}}}\,\,\,\,\xrightarrow{{}}\,\,\underset{(\text{Precipitate})}{\mathop{\overset{+}{\mathop{Ag}}\,\overset{-}{\mathop{Cl}}\,}}\,\,\downarrow +\overset{+}{\mathop{K}}\,\overset{\,-}{\mathop{N{{O}_{3}}}}\,\]
(viii) Electrovalent compounds show isomorphism.
(ix) Cooling curve of an ionic compound is not smooth, it has two break points corresponding to time of solidification.
(x) Ionic compounds show variable electrovalency due to unstability of core and inert pair effect.
According to Hess's law of constant heat summation, heat of formation of an ionic solid is net resultant of the above changes. \[\Delta {{H}_{f}}=\Delta {{H}_{\text{Subl}\text{.}}}+\frac{1}{2}\Delta {{H}_{\text{diss}\text{.}}}+IE-EA-U\]
(2) Characteristics of electrovalent compounds
(i) Electrovalent compounds are generally crystalline is nature. The constituent ions are arranged in a regular way in their lattice.
(ii) Electrovalent compounds possess high melting and boiling points. Order of melting and boiling points in halides of sodium and oxides of IInd group elements is as,
\[NaF>NaCl>NaBr>NaI,\]\[MgO>CaO>BaO\]
(iii) Electrovalent compounds are hard and brittle in nature.
(iv) Electrovalent solids do not conduct electricity. While electrovalent compounds in the molten state or in solution conduct electricity.
(v) Electrovalent compounds are fairly soluble in polar solvents and insoluble in non-polar solvents.
(vi) The electrovalent bonds are non-rigid and non-directional. Thus these compound do not show space isomerism e.g. geometrical or optical isomerism.
(vii) Electrovalent compounds furnish ions in solution. The chemical reaction of these compounds are known as ionic reactions, which are fast.
\[{{K}^{+}}C{{l}^{-}}+\overset{+}{\mathop{Ag}}\,\overset{-}{\mathop{N{{O}_{3}}}}\,\,\,\,\xrightarrow{{}}\,\,\underset{(\text{Precipitate})}{\mathop{\overset{+}{\mathop{Ag}}\,\overset{-}{\mathop{Cl}}\,}}\,\,\downarrow +\overset{+}{\mathop{K}}\,\overset{\,-}{\mathop{N{{O}_{3}}}}\,\]
(viii) Electrovalent compounds show isomorphism.
(ix) Cooling curve of an ionic compound is not smooth, it has two break points corresponding to time of solidification.
(x) Ionic compounds show variable electrovalency due to unstability of core and inert pair effect. 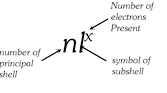 Some Unexpected Electronic Configuration
Some of the exceptions are important though, because they occur with common elements, notably chromium and copper.
\[Cu\] has 29 electrons. Its excepted electronic configuration is \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{9}}\] but in reality the configuration is \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{1}}3{{d}^{10}}\] as this configuration is more stable. Similarly \[Cr\] has the configuration of \[1{{s}^{2}}2{{s}^{2}}s{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{1}}3{{d}^{5}}\] instead of \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{4}}\].
Factors responsible for the extra stability of half-filled and completely filled subshells,
(i) Symmetrical distribution : It is well known fact that symmetry leads to stability. Thus the electronic configuration in which all the orbitals of the same subshell are either completely filled or are exactly half filled are more stable because of symmetrical distribution of electrons.
(ii) Exchange energy : The electrons with parallel spins present in the degenerate orbitals tend to exchange their position. The energy released during this exchange is called exchange energy. The number of exchanges that can take place is maximum when the degenerate orbtials (orbitals of same subshell having equal energy) are exactly half-filled or completely. As a result, the exchange energy is maximum and so it the stability.
Some Unexpected Electronic Configuration
Some of the exceptions are important though, because they occur with common elements, notably chromium and copper.
\[Cu\] has 29 electrons. Its excepted electronic configuration is \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{9}}\] but in reality the configuration is \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{1}}3{{d}^{10}}\] as this configuration is more stable. Similarly \[Cr\] has the configuration of \[1{{s}^{2}}2{{s}^{2}}s{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{1}}3{{d}^{5}}\] instead of \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{4}}\].
Factors responsible for the extra stability of half-filled and completely filled subshells,
(i) Symmetrical distribution : It is well known fact that symmetry leads to stability. Thus the electronic configuration in which all the orbitals of the same subshell are either completely filled or are exactly half filled are more stable because of symmetrical distribution of electrons.
(ii) Exchange energy : The electrons with parallel spins present in the degenerate orbitals tend to exchange their position. The energy released during this exchange is called exchange energy. The number of exchanges that can take place is maximum when the degenerate orbtials (orbitals of same subshell having equal energy) are exactly half-filled or completely. As a result, the exchange energy is maximum and so it the stability.  Note :
Note :
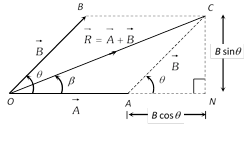 Special cases : \[R=A+B\] when q = 0o
\[R=A-B\] when q = 180o
\[R=\sqrt{{{A}^{2}}+{{B}^{2}}}\] when q = 90o
(2) Direction
\[\tan \beta =\frac{CN}{ON}=\frac{B\sin \theta }{A+B\cos \theta }\]
Special cases : \[R=A+B\] when q = 0o
\[R=A-B\] when q = 180o
\[R=\sqrt{{{A}^{2}}+{{B}^{2}}}\] when q = 90o
(2) Direction
\[\tan \beta =\frac{CN}{ON}=\frac{B\sin \theta }{A+B\cos \theta }\] 