question_answer 1) S.I. unit of magnetic flux is:
A)
tesla
done
clear
B)
forested
done
clear
C)
weber
done
clear
D)
gauss
done
clear
View Answer play_arrow
question_answer 2) A body of mass m is moving towards east and another body of equal mass is moving towards north. If after collision voth stick together, their P speed after collision would be :
A)
\[v\]
done
clear
B)
\[v/2\]
done
clear
C)
\[\sqrt{2\,v}\]
done
clear
D)
\[v/\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 3) A body of mass 1 kg is moving in a vertical r circular path of radius 1 m. The difference between the kinetic energies at its highest and lowest position is :
A)
20 J
done
clear
B)
10 J
done
clear
C)
\[4\sqrt{5}\] J
done
clear
D)
10\[(\sqrt{5}\]-1)J
done
clear
View Answer play_arrow
question_answer 4) Across each of two capacitors of capacitance\[1\mu F,\]a potential difference of 10 V is applied. Then positive plate of one is connected to the negative plate of the other, and negative plate of B6 one is connected to the positive plate of the R other. After contact:
A)
charge on each is zero
done
clear
B)
charge on each is same but non-zero
done
clear
C)
charge on each is different but non-zero
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 5) Magnification of a compound microscope is 30. Focal length of eye-piece is 5 cm and the image is formed at a distance of distinct vision of 25 cm. The magnification of the objective lens is :
A)
6
done
clear
B)
5
done
clear
C)
7.5
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 6) Kirchhoffs law of junction, \[\sum{I=0,}\] is based on :
A)
conservation of energy
done
clear
B)
conservation of charge
done
clear
C)
conservation of energy as well as charge
done
clear
D)
conservation of momentum
done
clear
View Answer play_arrow
question_answer 7) Calculate the amount of heat (in calories) required to convert 5g of ice at 0°C to steam at 100°C:
A)
3100
done
clear
B)
3200
done
clear
C)
3600
done
clear
D)
4200
done
clear
View Answer play_arrow
question_answer 8) A transverse wave is expressed as\[:y={{y}_{0}}\] sin \[2\pi fy.\] for what value of \[\lambda \] maximum particle velocity is equal to 4 times the wave velocity?
A)
\[{{y}_{O}}\pi /2\]
done
clear
B)
\[2y0\pi \]
done
clear
C)
\[{{y}_{0}}\pi \]
done
clear
D)
\[{{y}_{0}}\pi /4\]
done
clear
View Answer play_arrow
question_answer 9) Two bodies are thrown up at angles of 40° and 60°, respectively, with the horizontal. If both bodies attain same vertical height, then ratio of velocities with which these are thrown is :
A)
\[\sqrt{2/3}\]
done
clear
B)
\[2/\sqrt{3}\]
done
clear
C)
\[\sqrt{3/2}\]
done
clear
D)
\[\sqrt{3}/2\]
done
clear
View Answer play_arrow
question_answer 10) Charges 4Q, q and Q are placed along \[x\]-axis at positions \[=0,x=1/2\] and\[x=l,\]respectively. Find the value of q so that force on charge Q is zero:
A)
\[Q\]
done
clear
B)
\[\frac{Q}{2}\]
done
clear
C)
\[-\frac{Q}{2}\]
done
clear
D)
\[-Q\]
done
clear
View Answer play_arrow
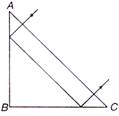
question_answer 11)
A ray falls on a prism ABC W = BC) and travels as shown in figure. The least value of refractive index of material of the prism, should be:
A)
\[1.5\]
done
clear
B)
\[\sqrt{2}\]
done
clear
C)
\[1.33\]
done
clear
D)
\[\sqrt{3}\]
done
clear
View Answer play_arrow
question_answer 12) Escape velocity from a planet is \[{{v}_{e.}}\] If its mass is increased to 8 times and its radius is increased to2 times, then the new escape velocity would be :
A)
\[{{v}_{e.}}\]
done
clear
B)
\[\sqrt{2}{{v}_{e.}}\]
done
clear
C)
\[2{{v}_{e.}}\]
done
clear
D)
\[2\sqrt{2}{{v}_{e.}}\]
done
clear
View Answer play_arrow
question_answer 13) A body takes time t to reach the bottom of an inclined plane of angle \[\theta \] with the horizontal. If the plane is made rough, time taken now is 2t. The coefficient of friction of the rough surface is :
A)
\[\frac{3}{4}\,\tan \,\theta \]
done
clear
B)
\[\frac{2}{3}\,\tan \,\theta \]
done
clear
C)
\[\frac{1}{4}\,\tan \,\theta \]
done
clear
D)
\[\frac{1}{2}\,\tan \,\theta \]
done
clear
View Answer play_arrow
question_answer 14) Two small charged spheres A and B have charges 10 \[\mu C\] and 40 \[\mu C\] respectively, and are held at a separation of 90 cm from each other. At what distance from A, electric intensity would be zero?
A)
22.5cm
done
clear
B)
18 cm
done
clear
C)
36 cm
done
clear
D)
30 cm
done
clear
View Answer play_arrow
question_answer 15) 50 tuning forks are arranged in increasing order of their frequencies such that each gives 4 beats/s with its previous tuning fork. If the frequency of the last fork is octave of the first, then the frequency of the first tuning fork is :
A)
200 Hz
done
clear
B)
204 Hz
done
clear
C)
196 Hz
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 16) In a cyclotron, if a deuteron can gain an energy of 40 MeV, then a proton can gain an energy of:
A)
40 MeV
done
clear
B)
80 MeV
done
clear
C)
20 MeV
done
clear
D)
60 MeV
done
clear
View Answer play_arrow
question_answer 17) Graph between velocity and displacement of particle, executing SHM is :
A)
a straight line
done
clear
B)
a parabola
done
clear
C)
a hyperbola
done
clear
D)
an ellipse
done
clear
View Answer play_arrow
question_answer 18) In the nuclear reaction,\[_{\text{72}}^{\text{180}}\text{ }\!\!\times\!\!\text{ }\xrightarrow{\text{- }\!\!\alpha\!\!\text{ }}\text{Y}\xrightarrow{\text{- }\!\!\beta\!\!\text{ }}\text{Z}\xrightarrow{\text{- }\!\!\alpha\!\!\text{ }}\text{A}\xrightarrow{\text{- }\!\!\gamma\!\!\text{ }}\] The atomic mass and number of P are, respectively:
A)
170, 69
done
clear
B)
172, 69
done
clear
C)
172, 70
done
clear
D)
170, 70
done
clear
View Answer play_arrow
question_answer 19) A radioactive substance has activity 64 times higher than the required normal level. If \[{{T}_{1/2}}=2h,\] then the time, after which it should be possible to work with it, is:
A)
16 h
done
clear
B)
6 h
done
clear
C)
10h
done
clear
D)
12h
done
clear
View Answer play_arrow
question_answer 20) An electron, moving in a uniform magnetic field of induction of intensity \[\mathbf{\vec{B}}\] has its radius directly proportional to:
A)
its charge
done
clear
B)
magnetic field
done
clear
C)
speed
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 21) The apparent frequency in Doppler's effect does not depend upon :
A)
speed of the observer
done
clear
B)
distance between observer and source
done
clear
C)
speed of the source
done
clear
D)
frequency from the source
done
clear
View Answer play_arrow
question_answer 22) Two simple pendulums whose lengths are 100 cm and 121 cm are suspended side by side. Their bobs are pulled together and then released After how many minimum oscillations of the longer pendulum, will the two be in phase again?
A)
11
done
clear
B)
10
done
clear
C)
21
done
clear
D)
20
done
clear
View Answer play_arrow
question_answer 23) If percentage change in current through a resistor is 1%, then the change in power through it would be :
A)
1%
done
clear
B)
2%
done
clear
C)
1.7%
done
clear
D)
0.5%
done
clear
View Answer play_arrow
question_answer 24) 3 identical bulbs are connected in series and these together dissipate a power P. If now the bulbs are connected in parallel, then the power dissipated will be :
A)
P/3
done
clear
B)
3P
done
clear
C)
9P
done
clear
D)
P/9
done
clear
View Answer play_arrow
question_answer 25) Acceleration due to gravity :
A)
decreases from equator to poles
done
clear
B)
decreases from poles to equator
done
clear
C)
is maximum at the centre of the earth
done
clear
D)
is maximum at the equator
done
clear
View Answer play_arrow
question_answer 26) Two bodies having masses \[{{m}_{1}}\] = 40g and \[{{m}_{2}}\] = 60g are attached to the ends of a string of negligible mass and suspended from massless pulley. The acceleration of the bodies is:
A)
1 nVs2
done
clear
B)
2m/s2
done
clear
C)
0.4 m/s2
done
clear
D)
4 m/s2
done
clear
View Answer play_arrow
question_answer 27) A block is moving up an inclined plane of inclination 60° with velocity of 20 m/s and stops after 2s. If \[g=20m/{{s}^{2}}\]then the approximate of value of coefficient of friction is :
A)
3
done
clear
B)
3.3
done
clear
C)
0.27
done
clear
D)
0.33
done
clear
View Answer play_arrow
question_answer 28) A coin placed on a rotating turntable just slips if it is placed at a distance of 8 cm from the centre. If angular velocity of the turntable is doubled, it will just slip at a distance of :
A)
1 cm
done
clear
B)
2 cm
done
clear
C)
4cm
done
clear
D)
8cm
done
clear
View Answer play_arrow
question_answer 29) When a man increases his speed by 2 m/s, he finds that his kinetic energy is doubled, the & original speed of the man is:
A)
\[2(\sqrt{2}-1)\]m/s
done
clear
B)
\[2(\sqrt{2}+1)\] m/s
done
clear
C)
4.5 m/s
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 30) A ball falling freely from a height of 4.9 m/s hits 3 a horizontal surface. If \[e=\frac{3}{4},\] then the ball will 4 hit the surface second time after:
A)
0.5s
done
clear
B)
1.5s
done
clear
C)
3.5s
done
clear
D)
3.4s
done
clear
View Answer play_arrow
question_answer 31) The distance between the sun and the earth be r then the angular momentum of the earth around the sun is proportional to :
A)
\[\sqrt{r}\]
done
clear
B)
\[{{r}^{3/2}}\]
done
clear
C)
\[r\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 32) A body executing SHM has its velocity 10 cm/s and 7 cm/s, when its displacements from the mean position are 3 cm and 4 cm respectively. The length of path is:
A)
10cm
done
clear
B)
9.5 cm
done
clear
C)
4cm
done
clear
D)
11.36cm
done
clear
View Answer play_arrow
question_answer 33) A 700 g solid cube having an edge of length 10 cm floats in water. The volume of cube outside water is :
A)
24 cm3
done
clear
B)
4.8 cm3
done
clear
C)
300 cm3
done
clear
D)
500 cm3
done
clear
View Answer play_arrow
question_answer 34) A one metre long steel wire of cross-sectional area 1 mm2 is extended by 1 mm. If \[\text{Y=2 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{11}}}\,\text{N/}{{\text{m}}^{\text{2}}}\text{,}\]then the work done is :
A)
0.1J
done
clear
B)
0.2J
done
clear
C)
0.3J
done
clear
D)
0.4J
done
clear
View Answer play_arrow
question_answer 35) The slit width, when a light of wavelength 6500Å is incident on a slit, if first minima for red light is at 30°, is :
A)
\[1\times {{10}^{-6}}m\]
done
clear
B)
\[5.2\times {{10}^{-}}^{6}m\]
done
clear
C)
\[1.3\times {{10}^{-}}^{6}m\]
done
clear
D)
\[26\times {{10}^{-}}^{6}m\]
done
clear
View Answer play_arrow
question_answer 36) A light source approaches the observer with velocity 0.5 c. Doppler's shift for light of wavelength \[5500\overset{o}{\mathop{\text{A}}}\,\] is :
A)
\[616\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[1833\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[5500\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[6160\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 37) A charged particle moves in an electric field from A to B, then from B to A:
A)
If W \[_{AB}>\,W{{ }_{AB}},\]then the field is conservative
done
clear
B)
If W \[_{AB}+\,W{{ }_{BA}}=0,\]then the field is conservative
done
clear
C)
If W \[_{AB}+\,W{{ }_{BA}}\]then the field is conservative
done
clear
D)
If W\[_{AB}=W{{ }_{BA,}}\] then the field is conservative
done
clear
View Answer play_arrow
question_answer 38) A solid metal sphere of radius 50 cm carries a charge \[25\times {{10}^{-10}}C.\]The electrostatic potential at a distance of 20 cm from the centre will be:
A)
25 V
done
clear
B)
15 V
done
clear
C)
35 V
done
clear
D)
45 V
done
clear
View Answer play_arrow
question_answer 39) Two plates (area = S) charged to +\[{{q}_{1,}}\] and +\[{{q}_{2,}}\]\[({{q}_{2}}<{{q}_{2}})\]brought closer to form a capacitor of capacitance C. The potential difference across the plates is :
A)
\[\frac{{{q}_{1}}-{{q}_{2}}}{2C}\]
done
clear
B)
\[\frac{{{q}_{1}}-{{q}_{2}}}{C}\]
done
clear
C)
\[\frac{{{q}_{1}}-{{q}_{2}}}{4C}\]
done
clear
D)
\[\frac{2({{q}_{1}}-{{q}_{2}})}{C}\]
done
clear
View Answer play_arrow
question_answer 40) What is the drift velocity of electrons if the current flowing through a copper wire of 1 mm diameter is 1.1 A- Assume that each atom of copper contributes one electron : (Given : density of Cu = 9 g/cm3 and atomic weight of Cu = 63)
A)
0.3 mm/s
done
clear
B)
0.5 mm/s
done
clear
C)
0.1 mm/s
done
clear
D)
0.2 mm/s
done
clear
View Answer play_arrow
question_answer 41) A uniform magnetic field is at right angle to the direction of motion of proton. As a result, the proton describes a circular path of radius 2.5 cm. If the speed of proton is doubled then the radius of the circular path will be :
A)
0.5 cm
done
clear
B)
2.5 cm
done
clear
C)
5.0 cm
done
clear
D)
7.5 cm
done
clear
View Answer play_arrow
question_answer 42) An electron accelerated through a potential difference enters into a uniform transverse magnetic field and experience a force F. If the accelerating potential is increased lo 2V, the electron in the same magnetic field will experience a force :
A)
\[F\]
done
clear
B)
\[F/2\]
done
clear
C)
\[\sqrt{2}F\]
done
clear
D)
\[F2\]
done
clear
View Answer play_arrow
question_answer 43) The certain amount of current when flowing in a properly set tangent galvanometer, produces a deflection of \[45{}^\circ C\]. The current be reduced by a factor of\[\sqrt{3,}\] the deflection would :
A)
decrease by \[30{}^\circ \]
done
clear
B)
decrease by \[15{}^\circ \]
done
clear
C)
decrease by \[15{}^\circ \]
done
clear
D)
increase by \[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 44) If a current of 3 A flowing in the primary coil is reduced to zero in 0.01 s then the induced emf in the secondary coil is 1500 V, the mutual inductance between the two coils is:
A)
0.5 H
done
clear
B)
5 H
done
clear
C)
1.5H
done
clear
D)
10 H
done
clear
View Answer play_arrow
question_answer 45) In order to obtain time constant of 10 s in an RC circuit containing a resistance of \[{{10}^{3}}\Omega ,\] the capacity of the condenser should be :
A)
\[10\,\mu F\]
done
clear
B)
\[100\,\mu F\]
done
clear
C)
\[100\,0\mu F\]
done
clear
D)
\[1000\,0\mu F\]
done
clear
View Answer play_arrow
question_answer 46) An \[\alpha \]-particle when accelerated through a potential difference of V volt has a wavelength\[\lambda .\] associated with it. In order to have same \[\lambda ,\] by what potential difference a proton must be accelerated?
A)
8 V
done
clear
B)
6 V
done
clear
C)
4V
done
clear
D)
12V
done
clear
View Answer play_arrow
question_answer 47) Hailstone at 0°C falls a from a height of 1 km on an insulating surface converting whole of its kinetic energy into heat. What part of it will melt?\[(g=10m/{{s}^{2}})\]
A)
\[\frac{1}{33}\]
done
clear
B)
\[\frac{1}{8}\]
done
clear
C)
\[\frac{1}{3}\times 10-4\]
done
clear
D)
All of it will melt
done
clear
View Answer play_arrow
question_answer 48) At \[27{}^\circ C\], a motor car tyre has a pressure of 2atmospheres. The temperature at which the tyre suddenly burst wilt be : (Given : \[{{\text{ }\!\!\gamma\!\!\text{ }}_{\text{air}}}\]= 1.4)
A)
246.1 K
done
clear
B)
250 K
done
clear
C)
246 K
done
clear
D)
248 K
done
clear
View Answer play_arrow
question_answer 49) A flat mirror revolves at a constant angular velocity making 2 revolutions/s. With what velocity will a light spot move along a spherical screen with a radius of 10 m if the mirror is at a centre of curvature of the screen?
A)
251.2 m/s
done
clear
B)
261/2 m/s
done
clear
C)
271.2 m/s
done
clear
D)
241/2 m/s
done
clear
View Answer play_arrow
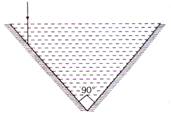
question_answer 50)
A vessel consists of two plane mirrors at right angles (as shown in figure). The vessel is filled with water. The total deviation in incident ray is:
A)
\[0{}^\circ \]
done
clear
B)
\[90{}^\circ \]
done
clear
C)
\[180{}^\circ \]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 51) If error in measurement of radius of sphere is 1%, what will be the error in measurement of volume?
A)
1%
done
clear
B)
1/3%
done
clear
C)
3%
done
clear
D)
10%
done
clear
View Answer play_arrow
question_answer 52) Consider the following equation of Bernoulli's theorem. \[P+\frac{1}{2}\rho {{v}^{2}}+\rho gh=K\] (constant) The dimensions of K/P are same as the of which of the following ?
A)
Thrust
done
clear
B)
Pressure
done
clear
C)
Angle
done
clear
D)
Viscosity
done
clear
View Answer play_arrow
question_answer 53) Why the dam of water reservoir is thick at the bottom?
A)
Quantity of water increases with depth
done
clear
B)
Density of water increases with depth
done
clear
C)
Pressure of water increases with depth
done
clear
D)
Temperature of water increases with depth
done
clear
View Answer play_arrow
question_answer 54) The length of second's pendulum is 1 m on earth. If mass and diameter of a planet is doubled than that of earth, then its length becomes:
A)
1 m
done
clear
B)
2 m
done
clear
C)
0.5 m
done
clear
D)
4 m
done
clear
View Answer play_arrow
question_answer 55) A beaker is completely filled with water at \[{{4}^{o}}C.\]It will overflow if:
A)
heated above 4°C
done
clear
B)
cooled below 4°C
done
clear
C)
both heated and cooled above and below 4°C respectively
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 56) A coin is dropped in a lift. It takes time\[{{t}_{1}}\]to reach the floor when lift is stationary. It late times \[{{t}_{2}}\] when life is moving up with constant acceleration. Then:
A)
\[{{t}_{1}}>{{t}_{2}}\]
done
clear
B)
\[{{t}_{2}}>{{t}_{1}}\]
done
clear
C)
\[{{t}_{1}}={{t}_{2}}\]
done
clear
D)
\[{{t}_{1}}>>{{t}_{2}}\]
done
clear
View Answer play_arrow
question_answer 57) An object is placed at a distance equal to focal length of convex mirror. If the focal length of the mirror be\[f,\]then the distance of the image from the pole of the mirror is :
A)
less than \[f\]
done
clear
B)
equal to \[f\]
done
clear
C)
more than \[f\]
done
clear
D)
infinity
done
clear
View Answer play_arrow
question_answer 58) What is the angle between the electric dipole moment and the electric field strength due to it on the equatorial line?
A)
\[0{}^\circ \]
done
clear
B)
\[90{}^\circ \]
done
clear
C)
\[180{}^\circ \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 59) A wire carrying current\[I\] and other carrying \[2I\]in the same direction produce a magnetic field B at the mid-point. What will be the field when \[2I\] wire is switched of?
A)
B / 2
done
clear
B)
2B
done
clear
C)
B
done
clear
D)
4B
done
clear
View Answer play_arrow
question_answer 60) The Fermi level of an intrinsic semiconductor is pinned at the centre of the band gap. The probability of occupation of the highest electron state in valence band at room temperature will be:
A)
zero
done
clear
B)
between zero and half
done
clear
C)
half
done
clear
D)
one
done
clear
View Answer play_arrow
question_answer 61) Which of the following is not an actinide?
A)
Curium
done
clear
B)
Californium
done
clear
C)
Uranium
done
clear
D)
Terbium
done
clear
View Answer play_arrow
question_answer 62) Europium is :
A)
s-block element
done
clear
B)
p-block element
done
clear
C)
d-block element
done
clear
D)
\[f-\]block element
done
clear
View Answer play_arrow
question_answer 63) For an electron, if the uncertainty in velocity is \[\Delta v,\]the uncertainty in its position\[(\Delta x)\]is given by:
A)
\[\frac{hm}{4\pi \Delta v}\]
done
clear
B)
\[\frac{4\pi }{hm\Delta v}\]
done
clear
C)
\[\frac{h}{4\pi m\Delta v}\]
done
clear
D)
\[\frac{4\pi m}{h\Delta v}\]
done
clear
View Answer play_arrow
question_answer 64) The reagent in Friedel-Craffs reaction is:
A)
pyridine
done
clear
B)
\[RCOCl\]
done
clear
C)
\[RCOOH\]
done
clear
D)
\[HCl\]
done
clear
View Answer play_arrow
question_answer 65) The\[{{K}_{sp}}\]of\[Mg{{(OH)}_{2}}\]is\[1\times {{10}^{-12}}.\text{ }0.01\text{ }M\] \[Mg{{(OH)}_{2}}\]will precipitate at the limited pH:
A)
3
done
clear
B)
9
done
clear
C)
5
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 66) Equation of Boyle?s law is:
A)
\[\frac{dp}{p}=-\frac{dV}{V}\]
done
clear
B)
\[\frac{dp}{p}=+\frac{dV}{V}\]
done
clear
C)
\[\frac{{{d}^{2}}p}{p}=-\frac{dV}{dT}\]
done
clear
D)
\[\frac{{{d}^{2}}p}{p}=+\frac{{{d}^{2}}V}{dT}\]
done
clear
View Answer play_arrow
question_answer 67) A radioactive sample is emitting 64 times radiations than non-hazardous limit. If its half-life is 2 hours, after what time it becomes non- hazardous?
A)
16h
done
clear
B)
12h
done
clear
C)
8h
done
clear
D)
4h
done
clear
View Answer play_arrow
question_answer 68) A metal surface is exposed to solar radiations:
A)
the emitted electrons have energy less than a maximum value of energy depending upon/ frequency of incident radiations
done
clear
B)
the emitted electrons have energy less than maximum value of energy depending upon intensity of incident radiations
done
clear
C)
the emitted electrons have zero energy
done
clear
D)
the emitted electrons have energy equal to energy of photons of incident light
done
clear
View Answer play_arrow
question_answer 69) Which of the following transitions have minimum wavelengths?
A)
\[{{n}_{4}}\to {{n}_{1}}\]
done
clear
B)
\[{{n}_{2}}\to {{n}_{1}}\]
done
clear
C)
\[{{n}_{4}}\to {{n}_{2}}\]
done
clear
D)
\[{{n}_{3}}\to {{n}_{1}}\]
done
clear
View Answer play_arrow
question_answer 70) Orbital is:
A)
circular path around the nucleus in which the electrons revolves
done
clear
B)
space around the nucleus where the probability of finding the electron is maximum
done
clear
C)
amplitude of electron wave
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 71) Number of unpaired electrons in\[M{{n}^{4+}}\]is :
A)
3
done
clear
B)
5
done
clear
C)
6
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 72) Which of the following sequence is correct as per Aufbau principle?
A)
\[35\text{ }<\text{ }3d\text{ }<\text{ }45\text{ }<\text{ }4p\]
done
clear
B)
\[1s\text{ }<\text{ }2p\text{ }<\text{ }4s\text{ }<\text{ }3d\]
done
clear
C)
\[2s\text{ }<\text{ }55\text{ }<\text{ }4p\text{ }<\text{ }Sd\]
done
clear
D)
\[2s\text{ }<\text{ }2p\text{ }<\text{ }3d\text{ }<\text{ }3p\]
done
clear
View Answer play_arrow
question_answer 73) Ionic compounds are formed most easily with:
A)
low electron affinity, high ionisation energy
done
clear
B)
high electron affinity, low ionisation energy
done
clear
C)
low electron affinity, low ionisation energy
done
clear
D)
high electron affinity, high ionisation energy
done
clear
View Answer play_arrow
question_answer 74) The enthalpy change\[(\Delta H)\]for the neutralization of M\[HCl\] by caustic potash in dilute solution at 298 K is:
A)
68 kJ
done
clear
B)
65 kJ
done
clear
C)
57.3 kJ
done
clear
D)
50 kJ
done
clear
View Answer play_arrow
question_answer 75) Which of the following is not hydrolysed?
A)
\[AsC{{l}_{3}}\]
done
clear
B)
\[P{{F}_{3}}\]
done
clear
C)
\[SbC{{l}_{3}}\]
done
clear
D)
\[N{{F}_{3}}\]
done
clear
View Answer play_arrow
question_answer 76) Which of the following gas is linear?
A)
\[C{{O}_{2}}\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
\[N{{O}_{2}}\]
done
clear
D)
\[S{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 77) \[N{{H}_{4}}COON{{H}_{2}}(s)(g)+C{{O}_{2}}(g,)\]if equlibrium pressure is 3 atm for the above reaction.\[{{K}_{p}}\]for the reaction is:
A)
4
done
clear
B)
27
done
clear
C)
4/27
done
clear
D)
1/27
done
clear
View Answer play_arrow
question_answer 78) Number of isomeric primary amines obtained from\[{{C}_{4}}{{H}_{11}}N\]are:
A)
3
done
clear
B)
4
done
clear
C)
5
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 79) If hydrogen electrode dipped in two solution of\[pH=3\]and\[pH=6\]and salt bridge is connected, the emf of resulting cell is:
A)
0.177V
done
clear
B)
0.3V
done
clear
C)
0.052V
done
clear
D)
0.104V
done
clear
View Answer play_arrow
question_answer 80) A radioactive nucleus will not emit:
A)
alpha and beta rays simultaneously
done
clear
B)
beta and gamma rays simultaneously
done
clear
C)
gamma and alpha rays
done
clear
D)
gamma rays only
done
clear
View Answer play_arrow
question_answer 81) In face centred cubic unit cell edge length is:
A)
\[\frac{4}{\sqrt{3}}r\]
done
clear
B)
\[\frac{4}{\sqrt{2}}r\]
done
clear
C)
\[2r\]
done
clear
D)
\[\frac{\sqrt{3}}{2}r\]
done
clear
View Answer play_arrow
question_answer 82) If the\[Z{{n}^{2+}}/Zn\] electrode is diluted to 100 times then the change in emf :
A)
increase of 59 mV
done
clear
B)
decrease of 59 mV
done
clear
C)
increase of 29.5 mV
done
clear
D)
decrease of 29.5 mV
done
clear
View Answer play_arrow
question_answer 83) Which of the following reactions end infinite time?
A)
Zero order
done
clear
B)
1st order
done
clear
C)
2nd order
done
clear
D)
3rd order
done
clear
View Answer play_arrow
question_answer 84) If equivalent conductance of 1M benzoic acid is\[12.8\text{ }oh{{m}^{-1}}c{{m}^{2}}\]and if the conductance of benzoate ion and\[{{H}^{+}}\]ion are 42 and 288.42\[oh{{m}^{-1}}c{{m}^{2}}\]respectively. Its degree of dissociation is:
A)
39%
done
clear
B)
3.9%
done
clear
C)
0.35%
done
clear
D)
0.039%
done
clear
View Answer play_arrow
question_answer 85) In which of the following reactions carbon-carbon bond formation takes place?
A)
Cannizaro
done
clear
B)
Reimer-Tiemann
done
clear
C)
HVZ reaction
done
clear
D)
Schmidt reaction
done
clear
View Answer play_arrow
question_answer 86) Which gives only mono-substituted product?
A)
o-dinitrobenzene
done
clear
B)
m-dinitrobenzene
done
clear
C)
p-dinitrobenzene
done
clear
D)
Nitrobenzene
done
clear
View Answer play_arrow
question_answer 87) Which of the following is most polarised?
A)
Kr
done
clear
B)
He
done
clear
C)
Ar
done
clear
D)
Xe
done
clear
View Answer play_arrow
question_answer 88) Order of boiling point is:
A)
\[HF>HI>HBr>HCl\]
done
clear
B)
\[HF>HBr>HI>HCl\]
done
clear
C)
\[HCl>HBr>HI>HF\]
done
clear
D)
\[HCl>HI>HBr>HF\]
done
clear
View Answer play_arrow
question_answer 89) If two substances A and B have\[P_{A}^{o}:P_{B}^{o}=1:2\] and have mole fraction in solution 1 : 2 then mole fraction of A in vapours:
A)
0.33
done
clear
B)
0.25
done
clear
C)
0.52
done
clear
D)
0.2
done
clear
View Answer play_arrow
question_answer 90) Order of hydrolysis for the following: (I)\[RCOCl\] (II)\[RCOOR\] (III)\[RCON{{H}_{2}}\] (IV)\[{{(RCO)}_{2}}O\]
A)
I > IV> II > III
done
clear
B)
I > II > III > IV
done
clear
C)
I > III > II > IV
done
clear
D)
IV > III > II > I
done
clear
View Answer play_arrow
question_answer 91) If an aqueous solution of glucose is allowed to freeze then crystal of which will be separated out first?
A)
Glucose
done
clear
B)
Water
done
clear
C)
Both and
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 92) \[CH\equiv CH\xrightarrow[{{H}_{2}}S{{O}_{4}}]{HgS{{O}_{4}}}\xrightarrow[{{H}_{2}}O]{C{{H}_{3}}MgBr}\xrightarrow{P/B{{r}_{2}}}\]
A)
\[C{{H}_{3}}CH(Br)C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Br\]
done
clear
C)
\[C{{H}_{2}}=CHBr\]
done
clear
D)
\[BrCH=CHC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 93) Number of bonds in benzene:
A)
\[6\sigma \]and\[3\pi \]
done
clear
B)
\[12\sigma \]and\[3\pi \]
done
clear
C)
\[3\sigma \]and \[12\pi \]
done
clear
D)
\[6\sigma \]and\[6\pi \]
done
clear
View Answer play_arrow
question_answer 94) If the\[{{V}_{rms}}\]is\[30{{R}^{1/2}}\]at\[27{}^\circ C\]then calculate the molar mass of gas in kilogram.
A)
1
done
clear
B)
2
done
clear
C)
4
done
clear
D)
0.001
done
clear
View Answer play_arrow
question_answer 95) If the enolate ion combines with carbonyl group of ester, we get:
A)
aldol
done
clear
B)
\[\alpha ,\beta -\] unsaturated ester
done
clear
C)
\[\beta -\]keto aldehyde
done
clear
D)
acid
done
clear
View Answer play_arrow
question_answer 96)
A)
enantiomers
done
clear
B)
diastereomers
done
clear
C)
meso compound
done
clear
D)
identical
done
clear
View Answer play_arrow
question_answer 97) The standard molar heat of formation of ethane,\[C{{O}_{2}}\]and water are respectively\[-\text{ }21.1,\]\[-\text{ }94.1\]and\[-68.3\]kcal. The standard molar heat of combustion of ethane will be:
A)
\[-372\text{ }kcal\]
done
clear
B)
\[162\text{ }kcal\]
done
clear
C)
\[-240\text{ }kcal\]
done
clear
D)
\[183.5\text{ }kcal\]
done
clear
View Answer play_arrow
question_answer 98) \[_{72}{{X}^{180}}\xrightarrow{2\alpha }\xrightarrow[{}]{\beta }{{\xrightarrow{\gamma }}_{z}}{{X}^{A}},\]Z and A are:
A)
69, 172
done
clear
B)
172, 69
done
clear
C)
180, 70
done
clear
D)
182, 68
done
clear
View Answer play_arrow
question_answer 99) \[A+2B\xrightarrow[{}]{{}}C+D,\]If \[-\frac{d[A]}{dt}=5\times {{10}^{-4}}mol\,{{L}^{-1}}{{s}^{-1}},\] then\[-\frac{d[B]}{dt}\] is:
A)
\[2.5\times {{10}^{-4}}mol\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
B)
\[50\times {{10}^{-4}}mol\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
C)
\[2.5\times {{10}^{-3}}mol\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
D)
\[1.0\times {{10}^{-3}}mol\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 100) Which of the following is paramagnetic?
A)
\[{{N}_{2}}\]
done
clear
B)
\[{{C}_{2}}\]
done
clear
C)
\[N_{2}^{+}\]
done
clear
D)
\[O_{2}^{2-}\]
done
clear
View Answer play_arrow
question_answer 101) Which of the following compounds will react with\[NaHC{{O}_{3}}\]solution to give sodium salt and carbon dioxide?
A)
Acetic acid
done
clear
B)
n-hexanol
done
clear
C)
Phenol
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 102) Which will give chiral molecule?
A)
\[C{{H}_{3}}COCl\xrightarrow{LiAl{{H}_{4}}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}CHO\xrightarrow[{{H}^{+}}/{{H}_{2}}O]{C{{H}_{3}}MgBr}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}CH{{C}_{2}}{{H}_{5}}\xrightarrow{Cu}\]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 103) Which is not a polymer?
A)
Sucrose
done
clear
B)
Enzyme
done
clear
C)
Starch
done
clear
D)
Teflon
done
clear
View Answer play_arrow
question_answer 104) Which statement is wrong for NO?
A)
It is anhydride of nitrous acid
done
clear
B)
Its dipole moment is 0.22 D
done
clear
C)
It forms dimer
done
clear
D)
It is paramagnetic
done
clear
View Answer play_arrow
question_answer 105) Which of the following reactions will not give propane?
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Cl\xrightarrow[{{H}_{2}}O]{Mg/ether}\]
done
clear
B)
\[C{{H}_{3}}COCl\xrightarrow[{{H}_{2}}O]{C{{H}_{3}}MgX}\]
done
clear
C)
\[C{{H}_{3}}CH\equiv C{{H}_{2}}\xrightarrow[C{{H}_{3}}COOH]{{{B}_{2}}{{H}_{6}}}\]
done
clear
D)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\xrightarrow{P/HI}\]
done
clear
View Answer play_arrow
question_answer 106) A compound\[A\to {{C}_{5}}{{H}_{10}}C{{l}_{2}}\]on hydrolysis gives \[{{C}_{5}}{{H}_{10}}O\]which reacts with\[NH{{ }_{2}}OH,\]forms iodoform, but does not give Fehling test. A is:
A)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-CH{{ }_{2}}-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{CH}}}\,\]
done
clear
D)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{CH}}\,-\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 107) In photography, sodium thiosulphate is used as:
A)
Completing agent
done
clear
B)
oxidizing agent
done
clear
C)
Reducing agent
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 108)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 109) There is no\[SS\]bond in:
A)
\[{{S}_{2}}O_{4}^{2-}\]
done
clear
B)
\[{{S}_{2}}O_{5}^{2-}\]
done
clear
C)
\[{{S}_{2}}O_{3}^{2-}\]
done
clear
D)
\[{{S}_{2}}O_{7}^{2-}\]
done
clear
View Answer play_arrow
question_answer 110) Which of the following is not a broad spectrum antibiotic?
A)
Tetracycline
done
clear
B)
Chloromycetin
done
clear
C)
Penicillin
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 111) Phenol and benzoic acid are distinguished by:
A)
\[NaOH\]
done
clear
B)
\[NaHC{{O}_{3}}\]
done
clear
C)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
D)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 112) Substance used as antiseptic for curing wounds is:
A)
equanil
done
clear
B)
iodine
done
clear
C)
proguanil
done
clear
D)
bithional
done
clear
View Answer play_arrow
question_answer 113) The IUPAC name of compound is: \[C{{H}_{2}}=CHCH{{(C{{H}_{3}})}_{2}}\]
A)
1-isopropylethylene
done
clear
B)
2-vinyl propane
done
clear
C)
3-methyl 1-butene
done
clear
D)
1, 1, dimethyl 1, 2-propene
done
clear
View Answer play_arrow
question_answer 114) Intermolecular hydrogen bonding is strongest in:
A)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
B)
\[HF\]
done
clear
C)
\[{{H}_{2}}O\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 115) \[CuS{{O}_{4}}\]does not react with:
A)
\[Fe\]
done
clear
B)
\[Ag\]
done
clear
C)
\[Zn\]
done
clear
D)
\[Mg\]
done
clear
View Answer play_arrow
question_answer 116) Cobalt-60 is used in the treatment of:
A)
leukaemia
done
clear
B)
thyroid disorders
done
clear
C)
cancer
done
clear
D)
pneumonia
done
clear
View Answer play_arrow
question_answer 117) Silicon is:
A)
semi-conductor
done
clear
B)
insulator
done
clear
C)
conductor
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 118) When lead nitrate is heated it produces:
A)
\[N{{O}_{2}}\]
done
clear
B)
NO
done
clear
C)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
D)
\[{{N}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 119) Which of the following test is not used for testing of proteins?
A)
Milton's test
done
clear
B)
Molisch's test
done
clear
C)
Biuret test
done
clear
D)
Ninhydrin test
done
clear
View Answer play_arrow
question_answer 120) Non-directional orbital is:
A)
\[4p\]
done
clear
B)
\[4d\]
done
clear
C)
\[4f\]
done
clear
D)
\[3s\]
done
clear
View Answer play_arrow
question_answer 121) Which of the following is not vestigial in man?
A)
Tail vertebrae
done
clear
B)
Nails
done
clear
C)
Nictitating membrane
done
clear
D)
Vermiform appendix
done
clear
View Answer play_arrow
question_answer 122) The chemical used in National Malaria Eradication Programme is:
A)
2, 4-D
done
clear
B)
BHC
done
clear
C)
DDT
done
clear
D)
Pyrethroid
done
clear
View Answer play_arrow
question_answer 123) A eukaryotic gene contains two kinds of base sequences. Which of these plays an important role in protein synthesis ?
A)
Introns
done
clear
B)
Exons
done
clear
C)
Both 'a' and 'b'
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 124) The number of hydrogen bonds between adenine and thymine in a DNA molecule is :
A)
two
done
clear
B)
three
done
clear
C)
four
done
clear
D)
eight
done
clear
View Answer play_arrow
question_answer 125) The enzyme, which combines with non-protein part to form a functional enzyme known as :
A)
co-enzyme
done
clear
B)
holoenzyme
done
clear
C)
apoenzyme
done
clear
D)
prosthetic group m
done
clear
View Answer play_arrow
question_answer 126) Which of the following enzyme digest protein in stomach ?
A)
Trypsin
done
clear
B)
Pepsin
done
clear
C)
Crepsin
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 127) Passive food ingestion in Amoeba is known as:
A)
import
done
clear
B)
invagination
done
clear
C)
circumfluence
done
clear
D)
circumvallation
done
clear
View Answer play_arrow
question_answer 128) The slime moulds are characterized by the presence of:
A)
elaters
done
clear
B)
pseudoelaters
done
clear
C)
capiltitium
done
clear
D)
capitulum
done
clear
View Answer play_arrow
question_answer 129) Ecdysone is secreted from :
A)
insecta
done
clear
B)
trematoda
done
clear
C)
nematoda
done
clear
D)
polycheta
done
clear
View Answer play_arrow
question_answer 130) In the life cycle of mosquito, comma-shaped stage is:
A)
larval stage
done
clear
B)
pupal stage
done
clear
C)
imago stage
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 131) Haemocoel is found in:
A)
Hydra and Aurelia
done
clear
B)
Taenia and Ascaris
done
clear
C)
cockroach and Pila
done
clear
D)
Balanoglossus and Herdmonia
done
clear
View Answer play_arrow
question_answer 132) The group of anamniota includes:
A)
reptiles and birds
done
clear
B)
birds and mammals
done
clear
C)
fishes and amphibians
done
clear
D)
reptiles, and mammals
done
clear
View Answer play_arrow
question_answer 133) The excretory material of bony fish is:
A)
urea
done
clear
B)
protein
done
clear
C)
ammonia
done
clear
D)
amino acid
done
clear
View Answer play_arrow
question_answer 134) Different colours of frog skin are controlled by:
A)
hormones
done
clear
B)
melanocytes
done
clear
C)
nervous system
done
clear
D)
both 'a' and 'c'
done
clear
View Answer play_arrow
question_answer 135) Blastula of frog has :
A)
blastopore
done
clear
B)
blastocoel
done
clear
C)
arahenteron
done
clear
D)
gastropore
done
clear
View Answer play_arrow
question_answer 136) Carotene pigment is found in the cells of:
A)
dermis
done
clear
B)
epidermis
done
clear
C)
adipose cell
done
clear
D)
both 'b' and 'c'
done
clear
View Answer play_arrow
question_answer 137) Debove's membrane is a layer of:
A)
muscular tissue
done
clear
B)
epithelial tissue
done
clear
C)
connective tissue
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 138) Achilles tendon is associated with :
A)
gluteus muscle
done
clear
B)
hamstring muscle
done
clear
C)
quadriceps muscle
done
clear
D)
gastrocnemius muscle
done
clear
View Answer play_arrow
question_answer 139) The leucocytes contain which of the following in large quantity ?
A)
basophils
done
clear
B)
neutrophils
done
clear
C)
eosinophils
done
clear
D)
monocytes
done
clear
View Answer play_arrow
question_answer 140) Which part of our body secreted the hormone secretin ?
A)
ileum
done
clear
B)
stomach
done
clear
C)
duodenum
done
clear
D)
oesophagus
done
clear
View Answer play_arrow
question_answer 141) During inspiration, the diaphragm :
A)
expands
done
clear
B)
shows no change
done
clear
C)
contracts and flattens
done
clear
D)
relaxes to become dome-saped
done
clear
View Answer play_arrow
question_answer 142) The oxygen toxicity is related with :
A)
blood poisoning
done
clear
B)
collapse of alveolar walls
done
clear
C)
failure of ventilation of lungs
done
clear
D)
both 'a' and 'b'
done
clear
View Answer play_arrow
question_answer 143) Cardiac output is determined by :
A)
heart rate
done
clear
B)
stroke volume
done
clear
C)
blood flow
done
clear
D)
both 'a' and 'b'
done
clear
View Answer play_arrow
question_answer 144) The important function of lymph is to :
A)
transport oxygen to the brain
done
clear
B)
transport carbon dioxide lo the lungs
done
clear
C)
return RBCs to the lymph nodes
done
clear
D)
return interstitial fluid to the blood
done
clear
View Answer play_arrow
question_answer 145) The lining of intestine and kidneys in humans is:
A)
keratinized
done
clear
B)
brush borde
done
clear
C)
ciliated
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 146) The yellow colour of urine is due to the presence of:
A)
urea
done
clear
B)
uric acid
done
clear
C)
urochrome
done
clear
D)
bilirubin
done
clear
View Answer play_arrow
question_answer 147) The leydig cells secrete :
A)
oestrogen
done
clear
B)
testosterone
done
clear
C)
progesterone
done
clear
D)
corticosterone
done
clear
View Answer play_arrow
question_answer 148) The function of pineal body is to :
A)
lighten the skin colours
done
clear
B)
control sexual behavior
done
clear
C)
regulates the period of puberty
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 149) Which of the following nerve is purely motor nerve ?
A)
vagus
done
clear
B)
facial
done
clear
C)
abducens
done
clear
D)
trigeminal
done
clear
View Answer play_arrow
question_answer 150) Which of the following part of a neuron is covered by fatty sheath ?
A)
axon
done
clear
B)
cyton
done
clear
C)
dendrite
done
clear
D)
node of Ranvier
done
clear
View Answer play_arrow
question_answer 151) Outgrowth developing along with hilum of the seed is :
A)
Plumule
done
clear
B)
Radicle
done
clear
C)
Strophiole
done
clear
D)
Perisperm
done
clear
View Answer play_arrow
question_answer 152) Removal of top fertile soil by wind or water is :
A)
Siltation
done
clear
B)
Soil erosion
done
clear
C)
Weathering of soil
done
clear
D)
Leaching
done
clear
View Answer play_arrow
question_answer 153) A fruit developed from a condensed inflorescence is :
A)
simple fruit
done
clear
B)
aggregate fruit
done
clear
C)
composite fruit
done
clear
D)
etaerio
done
clear
View Answer play_arrow
question_answer 154) Acid rain are produced by ;
A)
excess \[N{{O}_{3}}\]and \[S{{O}_{2}}\]from burning fossil fuels
done
clear
B)
excess production of \[N{{H}_{3}}\]by industry and coal gas
done
clear
C)
excess release of carbon monoxide by incomplete combustion
done
clear
D)
excess formation of \[C{{O}_{2}}\]by combustion and animal respiration
done
clear
View Answer play_arrow
question_answer 155) The principal cereal crop of India/Asia is :
A)
Sorghum
done
clear
B)
barley
done
clear
C)
wheat
done
clear
D)
rice
done
clear
View Answer play_arrow
question_answer 156) The stage of cell cycle when cell has undergone differentiation is:
A)
\[{{G}_{0}}\]
done
clear
B)
\[{{G}_{1}}\]
done
clear
C)
\[{{G}_{3}}\]
done
clear
D)
\[{{G}_{4}}\]
done
clear
View Answer play_arrow
question_answer 157) Principle protein of cillia and flagella is :
A)
globulin
done
clear
B)
fibrin
done
clear
C)
flagellin
done
clear
D)
tubulin
done
clear
View Answer play_arrow
question_answer 158) Photosynthetic bacteria have:
A)
pigment system I (only one pigment system)
done
clear
B)
pigment system II
done
clear
C)
both and
done
clear
D)
some other type
done
clear
View Answer play_arrow
question_answer 159) Major coffee producing state of India is :
A)
Tamil Nadu
done
clear
B)
Kerala
done
clear
C)
Kamataka
done
clear
D)
Andhra Pradesh
done
clear
View Answer play_arrow
question_answer 160) DNA chromosome replication takes place during:
A)
\[{{\text{G}}_{\text{1}}}\text{-}\]phase
done
clear
B)
\[{{\text{G}}_{\text{2}}}\text{-}\]phase
done
clear
C)
S-phase
done
clear
D)
Prophase
done
clear
View Answer play_arrow
question_answer 161) Active transport occurs:
A)
against concentration gradient and requires ATP
done
clear
B)
against concentration gradient and does not require ATP
done
clear
C)
along concentration gradient but require ATP
done
clear
D)
along concentration gradient but does not require ATP
done
clear
View Answer play_arrow
question_answer 162) Two or more species occupying same or overlapping areas are :
A)
sympatric
done
clear
B)
sibling
done
clear
C)
sub species
done
clear
D)
allopatric
done
clear
View Answer play_arrow
question_answer 163) Terminal cytochrome of respiratory chain which donates electrons to oxygen is :
A)
cytochrome b
done
clear
B)
cytochrome c
done
clear
C)
cytochrome\[{{a}_{1}}\]
done
clear
D)
cytochrome \[{{a}_{3}}\]
done
clear
View Answer play_arrow
question_answer 164) Dendrochronology is the study of:
A)
height of a tree
done
clear
B)
diameter of a tree
done
clear
C)
age of the tree by counting the number of annual rings in the main stem
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 165) A student wanrs to study metaphasic behavior of chromosomes in a living cell. The technique most suitable is:
A)
phase contrast microscope
done
clear
B)
x-ray microscope
done
clear
C)
cell fractionation
done
clear
D)
scanning electron microscope
done
clear
View Answer play_arrow
question_answer 166) Pratonema is found in the life cycle of:
A)
Spirogyra
done
clear
B)
Rhizopus
done
clear
C)
Escherichia
done
clear
D)
Funaria
done
clear
View Answer play_arrow
question_answer 167) Energy currency (reservoir) of the cells is:
A)
AMP
done
clear
B)
ATP
done
clear
C)
RNA
done
clear
D)
DNA
done
clear
View Answer play_arrow
question_answer 168) An open collateral bundle is one in which:
A)
xylem and phloem are seprated by cambium
done
clear
B)
xylem and phloem lie side by side
done
clear
C)
cambium occurs on the outside of bundle
done
clear
D)
cambium does not occur in the bundle
done
clear
View Answer play_arrow
question_answer 169) Agar-agar commonly used in bacterial cultures and medication is obtained from :
A)
Sargassum
done
clear
B)
Gelidium
done
clear
C)
Ulothrix
done
clear
D)
Ulva
done
clear
View Answer play_arrow
question_answer 170) Dikaryon formation is characteristic of:
A)
ascomycetes and basidiomycetes
done
clear
B)
phycomycetes and basidiomycetes
done
clear
C)
ascomycetes and phycomycetes
done
clear
D)
phycomycetes and zygomycetes
done
clear
View Answer play_arrow
question_answer 171) Most effective wavelength of light for photosynthesis is :
A)
green
done
clear
B)
violet
done
clear
C)
red
done
clear
D)
yellow
done
clear
View Answer play_arrow
question_answer 172) Divisions characteristic of korper-kappe theory are:
A)
anticlinal
done
clear
B)
T-type
done
clear
C)
periclinal
done
clear
D)
irregular
done
clear
View Answer play_arrow
question_answer 173) Prothallus of fern has :
A)
antheridia and archegonia on lower surface
done
clear
B)
antheridia and archegonia on upper surface
done
clear
C)
antheridia on upper surface and archegonia on lower
done
clear
D)
antheridia on lower surface and archegonia on upper surface
done
clear
View Answer play_arrow
question_answer 174) Male cone of Pmus possesses :
A)
mega sporophylls
done
clear
B)
microsporophyus
done
clear
C)
anthers
done
clear
D)
ligules
done
clear
View Answer play_arrow
question_answer 175) \[{{\text{C}}_{\text{4}}}\]plants, synthesis of sugars/final \[\text{C}{{\text{O}}_{\text{2}}}\]fixation occurs in :
A)
palisade cells
done
clear
B)
spongy cells
done
clear
C)
undifferentiated mesophyll cells
done
clear
D)
bundle sheath cells
done
clear
View Answer play_arrow
question_answer 176) Cook cambium and vascular cambium are :
A)
parts of secondary xylem and phloem
done
clear
B)
parts of pericycle
done
clear
C)
lateral meristems
done
clear
D)
apical meristems
done
clear
View Answer play_arrow
question_answer 177) First natural cytokinin was discovered by :
A)
Skoog and Miller
done
clear
B)
Letham
done
clear
C)
Benson and Calvin
done
clear
D)
Thimann and Went
done
clear
View Answer play_arrow
question_answer 178) Grassland with scattered trees is :
A)
savanna
done
clear
B)
deciduous forest
done
clear
C)
evergreen forest
done
clear
D)
tropical rain forest
done
clear
View Answer play_arrow
question_answer 179) Major role of minor elements inside living organisms is to act as :
A)
binder of cell structure
done
clear
B)
constituent of hormone
done
clear
C)
building blocks of important amino acids
done
clear
D)
cofactor of enzymes
done
clear
View Answer play_arrow
question_answer 180) epipetalous and syngenesious stamens occur in:
A)
Solanaceae
done
clear
B)
Brassicaceae
done
clear
C)
Fabaceae
done
clear
D)
Asteraceae
done
clear
View Answer play_arrow
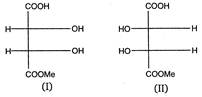 (I) and (II) are:
(I) and (II) are:
