Category : 9th Class
Atoms and Molecules
Chapter Overview
Around 500 B.C. an Indian philosopher Maharishi Kanda, said in his Darshan that if we go on dividing matter, we shall get smaller and smaller particles. A stage would come beyond which further division will not be possible. He named these particles as TAJRMANLT. This concept was further elaborated by another Indian philosopher, Pakudha Katya an. Katya an said, these particles normally exist in a combined form which gives us various forms of matter.
Around the same era, sin ancient Greek philosopher Democritus (460 – 370 B.C.) and Leucippus suggested that if we go on dividing matter, a stage will came when further division of particles will not be possible. Democritus called these individual particles 'atoms' (which means indivisible).
These ideas were based on philosophical considerations.
In this chapter, we shall study about atom and molecules and related aspects, like atomic and molecular masses, mole concept and molar masses. We shall also learn how to write chemical formula of a compound.
There are two main laws of chemical combinations:
(i) Law of conservation of mass,
(ii) Law of definite or constant proportions.
Lavoisier gave the Law of Conservation of Mass as:
In every chemical reaction, total masses of all the reactants is equal to the masses of all the products.
\[\Rightarrow \]Total mass of the substances before the reaction = Total mass of the substances after the reaction
For example, in the reaction of hydrogen \[({{H}_{2}})\]and chlorine \[(C{{l}_{2}})\]represented. Here 2g of
\[{{H}_{2}}\] reacts with 71g of \[C{{l}_{2}}\]to give 73 g of HCl.
|
|
\[{{H}_{2}}\]+ |
\[C{{l}_{2}}\]\[\to \] |
\[2HCl\] |
|
Molecular mass |
2 |
71 |
\[2\times 36.5=73\] |
Here molecular mass data indicates that the mass of the reactants is equal to the mass of the products i.e., the total mass is conserved in the reaction.
Law of Constant Proportions
In a given chemical compound, the proportions by mass of the elements that compose it are fixed, independent of the origin of the compound or its mode of preparation.
In pure water, for instance, the ratio of mass of hydrogen to mass of oxygen is always 1 : 8 irrespective of the source of water. In other words, pure water contains \[11\cdot 11%\]of hydrogen and \[88\cdot 89%\]of oxygen by mass, whether water is obtained from well, river or from a pond. Thus, if \[9\cdot 0\]g of water is decomposed, \[1\cdot 0\]g of hydrogen and \[8\cdot 0\] g of oxygen is always obtained.
We take another example of compound calcium oxide (CaO) which can be prepared by the following three independent methods:
(a) \[CaC{{O}_{3}}\to CaO+C{{O}_{2}}\]
(b) \[Ca{{(OH)}_{2}}\to CaO+{{H}_{2}}O\]
(c) \[2Ca{{(N{{O}_{3}})}_{2}}\to 2CaO+4N{{O}_{2}}+{{O}_{2}}\]
The analysis of calcium oxide thus, prepared by different methods and different sources, shows that it contains the elements calcium and oxygen only, and the ratio by mass of calcium to oxygen is always fixed that is 5 : 2.
In 1808, Dalton published a new system of chemical philosophy in which the following statements comprise the atomic theory of matter:
An atom is the smallest particle of an element that retains its (element's) chemical properties.
Atoms are very small, they are smaller than anything that we can imagine or compare with. In order to have a feeling of size of an atom you can consider this example:
One teaspoon of water (about 1 ml) contains about three times as many atoms as Atlantic ocean contains teaspoons of water.
For all practical purposes atom is taken as spherical in shape and that is why we talk of its radius. Since size of atom is extremely small and invisible to our eyes, we adopt a scale of nanometer \[(1nm={{10}^{-9}}m)\]to express its size. Their radii are of the order of\[{{10}^{-10}}m\].
Table 3.1: Relative Sizes
|
Radii (in m) |
Example |
| \[{{10}^{-10}}\] |
Atom of hydrogen |
| \[{{10}^{-9}}\] |
Molecule of water |
| \[{{10}^{-8}}\] |
Molecule of haemoglobin |
| \[{{10}^{-4}}\] |
Grain of sand |
| \[{{10}^{-2}}\] |
Ant |
| \[{{10}^{-1}}\] |
Watermelon |
5. Modern Day Symbols of atoms of Different Elements
Dalton was the first scientist to represent the various elements by definite symbols. These symbols also represented one atom of that element. When Dalton used a symbol for an element he also meant a definite quantity of that element that is one atom of that element. Notations of some of them given by Dalton are given below:
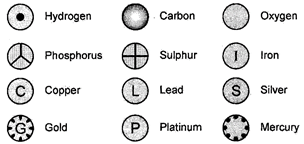
Fig. 3.1: Symbols for some elements as proposed by Dalton
Table 3.2 Symbols of Some Common Elements
|
Modern Name |
Symbol |
Latin or Greek Name |
|
Aluminum |
Al |
|
|
Antimony |
Sb |
Stibium (Latin) |
|
Argon |
Ar |
Agron (Greek) |
|
Arsenic |
As |
|
|
Barium |
Ba |
Barys Greek) |
|
Boron |
B |
|
|
Bromine |
Br |
Bromos (Greek) |
|
Calcium |
Ca |
Clax (Latin) |
|
Carbon |
C |
Carbonium (Latin) |
|
Chlorine |
Cl |
Chrom (Greek) |
|
Chromium |
Cr |
Chrom (Greek) |
|
Cobalt |
Co |
Co bold (German) |
|
Copper |
Cu |
Cobold (German) |
|
Fluorine |
F |
Fluo (Latin) |
|
Gold |
Au |
Aurum (Latin) |
|
Hydrogen |
H |
Hydrogenium (Latin) |
|
Iodine |
I |
Iodes (Greek) |
|
Iron |
Fe |
Ferrum (Latin) |
|
Krypton |
Kr |
Kryptos (Greek) |
|
Lead |
Pb |
Plumbum (Latin) |
|
Magnesium |
Mg |
|
|
Molybdenum |
Mo |
Molybdos (Greek) |
|
Mercury |
Hg |
Hydrargyrum (Latin) |
|
Neon |
Ne |
Neos (Greek) |
|
Nickel |
Ni |
|
|
Nitrogen |
N |
Nitrogenium (Latin) |
|
Oxygen |
O |
Oxygenium (Latin) |
|
Phosphorus |
P |
Phosphoros (Greek) |
|
Polonium |
Po |
|
|
Potassium |
K |
Kalium (Latin) |
|
Platinum |
Pt |
|
|
Selenium |
Se |
Selene (Greek) |
|
Silicon |
Si |
Silex (Latin) |
|
Silver |
Ag |
Argentum (Latin) |
|
Sodium |
Na |
Natrium (Latin) |
|
Strontium |
Sr |
|
|
Sulphur |
S |
Sulfur (Latin) |
|
Tantalum |
Ta |
Tantalos (Greek) |
|
Tin |
Sn |
Stannum (Latin) |
|
Titanium |
Ti |
Titan (Latin) |
|
Tungsten |
W |
Wolfram (Latin) |
|
Uranium |
U |
|
|
Vanadium |
V |
|
|
Xenon |
Xe |
Xenon (Greek) |
|
Zine |
Zn |
Zink (Greek) |
Now the names of elements are approved by the International Union of Pure and Applied Chemistry (IUPAC). For example, the symbol of the yet to be discovered element with the atomic number 117 is Uus (Ununseptium).
Definition of Symbol: The symbol of an element is an abbreviation for the full name of the element.
Significance of the symbol of an Element
The mass of an atom of an element is called its atomic mass. The atom is very small, therefore, its mass is also very small.
Table 3.3: Atomic mass of some common elementsb
|
Elements |
Symbol |
Mass (u) |
Elements |
Symbol |
Mass (u) |
|
Aluminium |
Al |
26.93 |
Magnesium |
Mg |
24.31 |
|
Argon |
Ar |
39.95 |
Manganese |
Mn |
54.94 |
|
Arsenic |
As |
74.92 |
Mercury |
Hg |
200.59 |
|
Barium |
Ba |
137.34 |
Neon |
Ne |
20.18 |
|
Boron |
B |
10.81 |
Nickel |
Ni |
58.71 |
|
Bromine |
Br |
79.91 |
Nitrogen |
N |
14.01 |
|
Caesium |
Cs |
132.91 |
Oxygen |
0 |
16.00 |
|
Calcium |
Ca |
40.08 |
Phosphorus |
P |
30.97 |
|
Carbon |
C |
12.01 |
Platinum |
Pt |
195.09 |
|
Chlorine |
d |
35-45 |
Potassium |
K |
39.1 |
|
Chromium |
Cr |
52.00 |
Radon |
Rn |
(222)** |
|
Cobalt |
Co |
58.93 |
Silicon |
Si |
23.09 |
|
Copper |
Cu |
63.56 |
Silver |
Ag |
107.87 |
|
Fluorine |
F |
19.00 |
Sodium |
Na |
23.00 |
|
Gold |
Au |
196.97 |
Sulphur |
S |
32.06 |
|
Helium |
He |
4.00 |
Tin |
Sn |
118.69 |
|
Hydrogen |
H |
1.008 |
Titanium |
Ti |
47.88 |
|
Iodine |
I |
126.90 |
Tungsten |
W |
183.85 |
|
Iron |
Fe |
55.85 |
Uranium |
U |
238.03 |
|
Lead |
Pb |
207.19 |
Vanadium |
V |
50.94 |
|
Lithium |
Li |
6.94 |
Xenon |
Xe |
131.30 |
|
|
|
|
Zinc |
Zn |
65.37 |
7.
A molecule is an aggregate of two or more than two atoms of the same or different elements in a definite arrangement.
These atoms are held together by chemical forces or chemical bonds.
(i) A molecule of a substance shows all chemical properties of that substance.
(ii) To describe the chemical composition of a molecule we take the help of symbols of elements and formulas.
Chemical formula of molecule
These are of two types:
(i) Molecular formulae,
(ii) Empirical formulae.
The symbolic representation of a molecule of a substance representing the actual number of various atoms present in it is called molecular formula. For example, the molecular formula of carbon dioxide is \[C{{O}_{2}}\], containing one atom of carbon and two atoms of oxygen.
The number of atoms of all the elements present in a molecule of a substance is known as atomicity of that molecule.
Table 3.4: Atomicity of some elements
|
Types of Element |
Name |
Atomicity |
|
Non-Metal |
Argon |
Monoatomic |
|
|
Helium |
Monoatomic |
|
|
Oxygen |
Diatomic |
|
|
Hydrogen |
Diatomic |
|
|
Nitrogen |
Diatomic |
|
|
Chlorine |
Diatomic |
|
|
Phosphorus |
Tetra-atomic |
|
|
Sulphur |
Poly-atomic |
Molecules of Elements
Oxygen molecule is made of two atoms of oxygen and therefore, it is a diatomic molecule (represented by \[{{O}_{2}}\]), hydrogen, nitrogen, fluorine, chlorine, bromine and iodine are other examples of diatomic molecules and are represented as \[{{H}_{2}},{{N}_{2}},{{F}_{2}},C{{l}_{2}},B{{r}_{2}}\]and \[{{I}_{2}}\]respectively.
Significance of Molecular formula
(i) It indicates the number of various atoms present in one molecule of the compound.
(ii) Molecular formula gives the number of gram-atoms of each element present in one- mole of the compound.
(iii) Mass of each element present in one mole of the compound can be found out from the molecular formula.
(iv) It indicates the names of various elements present in the compound.
(v) Relative molecular mass of the compound can be calculated from the molecular formula.
Molccules of Compounds
Molecules of compounds are composed of more than one kind of atoms. A familiar example is of water molecule which is composed of more than one kind of atoms. In one water molecule, there are two atoms of hydrogen and one atom of oxygen. It is represented as \[{{H}_{2}}O\]. A molecule of ammonia consists of one nitrogen atom and three hydrogen atoms. A molecule of ethyl alcohol \[({{C}_{2}}{{H}_{5}}OH)\] is composed of nine atoms (2 atoms of carbon, 6 atoms of hydrogen and 1 atom of oxygen).
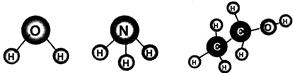
Fig. 3.2: Molecules of water, ammonia and ethyl alcohol
Table 3.5: Molecules of some compounds
|
Compound |
Combining Elements |
Ratio by Mass |
|
Water |
Hydrogen, Oxygen |
1:8 |
|
Ammonia |
Nitrogen, Hydrogen |
14:8 |
|
Carbon dioxide |
Carbon, Oxygen |
3:8 |
Ion is a charged chemical particle. The charged species formed when an atom gains 01 loses electron is called 'ion'. An ion can be positively or negatively charged. A positively charged ion is called cation and a negatively charged ion is called anion. A cation is formed when an atom loses one or more electrons. For example, when the potassium atom (K) loses one electron, the potassium ion\[({{K}^{+}})\]is formed.
|
K (solid) |
|
\[{{e}^{-}}\] |
|
\[{{K}^{+}}\](cation) |
|
Potassium Atom |
|
|
|
Potassium Ion |
When the chlorine atom (Cl) gains one electron, the chloride ion \[(C{{l}^{-}})\]is formed.
|
Cl (gas) |
|
\[{{e}^{-}}\] |
|
\[C{{l}^{-}}\](anion) |
|
Chlorine Atom |
|
|
|
Chlorine Ion |
Examples of cations:\[{{H}^{+}},L{{i}^{+}},N{{a}^{+}},{{K}^{+}},A{{g}^{+}},C{{a}^{2+}},B{{a}^{2+}},F{{e}^{3+}}\]
Examples of anions: \[C{{l}^{-}},B{{r}^{-}},{{I}^{-}},{{O}^{2-}},{{S}^{2-}},C{{O}_{2}}^{2-}\]etc.
A group of atoms carrying some charge is called a polyatomic ion. The examples of polyatomic ions are\[N{{H}_{4}}^{+},PC{{l}_{4}}^{+},C{{O}_{2}}^{2-},N{{O}_{2}}^{-},N{{O}_{3}}^{-},S{{O}_{4}}^{2-}\]etc.
An ionic compound contains cations and anions. The examples of ionic compounds are; \[NaCl,KCl,N{{a}_{2}}S{{O}_{4}},{{K}_{2}}S{{O}_{4}},MgS{{O}_{4}},AlP{{O}_{4}}\]etc. An ionic compound is formed when a metallic element reacts with a non-metallic element.
For example, when potassium metal reacts with chlorine gas the ionic compound, potassium chloride is formed. During the reaction of potassium metal and chlorine gas, one potassium atom loses one electron and one chlorine atom gains one electron. Thus, one electron is transferred from the potassium atom to the chlorine atom.
|
K (solid) |
|
\[\to \] | \[{{K}^{+}}\]\[+\]\[{{e}^{-}}\] |
|
(metal) |
|
\[\to \] |
|
|
Cl (gas) |
\[+{{e}^{-}}\] |
\[\to \] | \[C{{l}^{-}}\] |
|
(non-metal) |
|
\[\to \] |
|
|
\[{{K}^{+}}\] |
\[+C{{l}^{-}}\] |
\[\to \] |
\[K+C{{l}^{-}}\] |
The elements are represented by their symbols (e.g. H for hydrogen, Na for sodium).
Similarly, a compound is also represented by a shorthand notation known as formula or chemical formula. The formula of a compound indicates:
(i) elements constituting the compound and
(ii) number of each constituent element. In other words, the formula of a compound also represents its chemical composition.
Empirical formula of a compound is the simplest formula which gives the simplest ratio in whole numbers between the number of atoms of different elements present in one molecules of the compound.
For example, the empirical formula of benzene is CH. It indicates that the simplest ratio between the carbon and hydrogen atoms in its molecule is 1: 1 whereas, its actual formula is\[{{C}_{6}}{{H}_{6}}\]. Therefore, the empirical formula of the benzene having molecular formula of\[{{C}_{6}}{{H}_{6}}\] is CH.
Empirical formula of a compound does not indicate the actual number of atoms of the elements present in the compound. It only gives the simplest whole number ratio between the number of atoms of all the elements present in the compound. Empirical formula mass is the sum of the atomic masses of various elements representing the empirical formula. Thus, empirical formula mass of benzene is 12 + 1 = 13.
Molecular Formula \[=n\times \]Empirical formula where n is an integer.
Every element has a definite capacity to combine with other elements. This combining capacity of an element is called its Valency.
Valency of few elements are given ahead in Table 3.6.
Table 3.6: Valency of elements
|
Elements |
Symbol |
Valency |
Elements |
Symbol |
Valency |
|
Hydrogen |
H |
1 |
Phosphorus |
P |
5 |
|
Oxygen |
O |
2 |
Sodium |
Na |
1 |
|
Carbon |
C |
4 |
Magnesium |
Mg |
2 |
|
Nitrogen |
N |
3 |
Calcium |
Ca |
2 |
|
Chlorine |
Cl |
1 |
Aluminium |
Al |
3 |
|
Bromine |
Br |
1 |
Iron |
Fe |
2 |
|
Iodine |
I |
1 |
Barium |
Ba |
2 |
Formulation of an ionic compound is easy when we know charge of cation and anion.
Remember, in an ionic compound, sum of the charge of cation and anion should be equal to zero.
A few examples of cations and anions with their charges are provided in Table 3-7. below:
Table 3.7: Charges of some common cations and anions which form ionic compounds
|
Anions |
Charge |
Cations |
Charge |
| Chloride ion, \[C{{l}^{-}}\] |
- 1 |
Potassium ion, |
+1 |
|
Nitrate ion,\[N{{O}_{3}}^{-}\] |
- 1 |
Sodium ion, |
+1 |
|
Hydroxide ion,\[O{{H}^{-}}\] |
- 1 |
Ammonium ion, |
+1 |
|
Bicarbonate ion,\[HC{{O}_{3}}^{-}\] |
- 1 |
Magnesium ion, |
+2 |
|
Nitrite ion,\[N{{O}_{2}}^{-}\] |
- 1 |
Calcium ion, |
+2 |
|
Acetate ion,\[C{{H}_{3}}CO{{O}^{-}}\] |
- 1 |
Lead ion, |
+2 |
|
Bromide ion,\[B{{r}^{-}}\] |
- 1 |
Iron ion (Ferrous), |
+2 |
|
Iodide ion,\[{{I}^{-}}\] |
- 1 |
Zinc ion, |
+2 |
|
Sulphite ion,\[S{{O}_{2}}^{2-}\] |
- 2 |
Copper ion (cupric), |
+2 |
|
Carbonate ion,\[C{{O}_{3}}^{2-}\] |
- 2 |
Mercury ion (Mercuric), |
+2 |
|
Sulphate ion\[S{{O}_{4}}^{2-}\] |
- 2 |
Iron (Ferric) ion, |
+3 |
|
Sulphide ion, \[{{S}^{2-}}\] |
- 2 |
Aluminium ion, |
+3 |
|
Phosphate ion,\[P{{O}_{4}}^{3-}\] |
- 3 |
|
|
|
Potassium ion,\[{{K}^{+}}\] |
-1 |
|
|
|
Sodium ion,\[N{{a}^{+}}\] |
-1 |
|
|
Suppose you have to write formula of sodium sulphate which is made of \[N{{a}^{+}}\]and \[S{{O}_{4}}^{2-}\]ions. For this the positive and negative charge can be crossed over to give subscripts. The purpose of this crossing over the charges is to find the number of ions required to equate the I number of positive and negative charges.
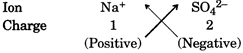
This gives the formula of sodium sulphate as\[N{{a}_{2}}S{{O}_{4}}\]. We can check the charge balance as follows:
\[\left. \begin{align}
& 2N{{a}^{+}}=2\times (+1)=+2 \\
& 1SO_{4}^{2-}=1\times (-2)=-2 \\
\end{align} \right]=0\]
Thus, the compound, Na2S04 is electrically neutral.
Now it is clear that digit showing charge of cation goes to anion and digit showing charge of anion goes to cation.
Some More Examples
|
1. |
Formula of magnesium chloride Symbol Charge Formula |
|
|
2. |
Formula for calcium oxide Symbol Charge Formula |
|
|
3. |
Formula for aluminium oxide Symbol Charge Formula |
|
|
4. |
Formula of sodium nitrate Symbol Charge Formula |
|
|
5. |
Formula of calcium hydroxide Symbol Charge Formula |
|
|
6. |
Formula of sodium carbonate Symbol Charge Formula |
|
|
7. |
Formula of ammonium sulphate Symbol Charge Formula |
|
|
8. |
Formula of magnesium nitrate Symbol Charge Formula |
|
Molecular formula of a compound is normally used for determining the molecular mass of that substance.
Since the mass of a molecule is very small, it is expressed relative to the mass of a carbon atom(C-12).
Relative Molecular Mass
The mass of a molecule of a substance compared with one twelfth mass of a carbon-12 atom is called relative molecular mass of that substance.
Relative molecular mass \[\text{=}\frac{\text{Mass of one atom of the substance}}{\frac{1}{12}\times \text{Mass of one atom of C-12}}\]
For example, the relative molecular mass of oxygen \[({{O}_{2}})\]is 32. It means that a molecule of oxygen is\[\frac{32}{12}\] times as heavy as an atom of C-12. That is, molecular mass of oxygen is 32 times greater than \[\frac{1}{12}\]th the mass of C-atom.
The percentage composition of a compound is the mass of each element of the compound present in 100 g of that compound i.e., the mass percentage of each element present in the compound.
Calculation of mass percentage:
(i) When the masses of compound and each element are given:
Mass of element in the given mass
Mass percentage of an element \[(x)=\frac{\text{of}\,\text{the}\,\text{compound}}{\text{Total}\,\text{mass}\,\text{of}\,\text{compound}}\times 100\]
Total mass of the compound
(ii) When the formula of the compound and the atomic masses of the elements are given:
Total mass of element in one
Mass percentage of an element \[\text{(y)=}\frac{\text{molecule}\,\text{of}\,\text{the}\,\text{compound}}{\text{Molecular}\,\text{mass}\,\text{of}\,\text{the}\,\,\text{compound}}\text{ }\!\!\times\!\!\text{ 100}\]
A mole is the amount of substance that contains as many elementary entities (atoms, molecules, formula unit or other fundamental particles) as there are atoms in exactly \[0\cdot 012\] kg of carbon-12 isotope.
In simple words, mole is the number of atoms in exactly \[0\cdot 012\]kg (12 grams) of carbon-12 element.
Mole is a scientist's counting unit like dozen. By using mole, scientists count atom and molecules in a given substance.
Now it is experimentally found that the number of atoms contained in exactly 12 g of C-12 is 602,200000000000000000000 or\[6.02\times {{10}^{23}}\]. This number is called Avogadro's number in honour of Amedeo Avogadro, an Italian lawyer and physicist. When this number is divided by 'mole' it becomes a constant and is known as Avogadro's constant denoted by symbol \[{{N}_{0}}=6.02\times {{10}^{23}}mo{{l}^{-1}}\]
Mass of one mole of a substance is called its molar mass: A substance may be an element or a compound. Mass of one mole of atoms of oxygen means mass of \[6\cdot 02\times {{10}^{23}}\]atoms of oxygen.
It is found that one mole atoms of oxygen weighs 16.0 g.
Molar mass is always expressed in the unit of g/mol or g \[mo{{l}^{-1}}\].
For example,
Molar mass of nitrogen \[({{N}_{2}})=28gmo{{l}^{-1}}\]
Molar mass of chlorine \[(C{{l}_{2}})=71gmo{{l}^{-1}}\]
The quantity of an element in gram equal to the relative atomic mass is termed as the gram-atomic mass (or simply gram-atom) of the element. The mass of 1 mole of atoms of an element in grams is equal to its relative atomic mass. Therefore, we can say that gram atomic mass or gram-atom is the mass of one Avogadro's number of atoms expressed in grams.
1 gram atomic mass = 1 gram – atom
= mass of \[6\cdot 023\times {{10}^{23}}\] atoms of the substance.
Gram-molecular Mass of a Compound
The quantity of a compound in gram equal to its relative molecular mass is termed as the gram-molecular mass or gram-mole or simply mole of the compound.
1 gram molecular mass = 1 gram – molecule
= Mass of \[=n\times Empirical\text{ }formula\]molecules of the substance
Chapter at a Glance
The charge an one mole of electrons is called one Faraday which is equal to 96500C.
You need to login to perform this action.
You will be redirected in
3 sec
