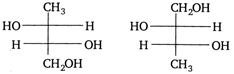question_answer 1) One kg of copper is drawn into a wire of 1 mm diameter and a wire of 2 mm diameter. The resistance of the two wires will be in the ratio
A)
2:1
done
clear
B)
1:2
done
clear
C)
16:1
done
clear
D)
4:1
done
clear
View Answer play_arrow
question_answer 2) An electrical cable having a resistance of \[0.2\,\Omega \]delivers 10 kW at 200 V DC to a factory. What is the efficiency of transmission?
A)
65%
done
clear
B)
75%
done
clear
C)
85%
done
clear
D)
95%
done
clear
View Answer play_arrow
question_answer 3) A wire of resistance \[5\Omega \]is drawn out so that its new length is 3 times its original length. What is the resistance of the new wire?
A)
\[45\,\Omega \]
done
clear
B)
\[15\,\Omega \]
done
clear
C)
\[5/3\,\,\Omega \]
done
clear
D)
\[5\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 4) Two identical cells each of emf E and internal resistance r are connected in parallel with an-external resistance R. To get maximum power developed across R, the value of R is
A)
\[R=\frac{r}{2}\]
done
clear
B)
\[R=r\]
done
clear
C)
\[R=\frac{r}{3}\]
done
clear
D)
\[R=2r\]
done
clear
View Answer play_arrow
question_answer 5) To write the decimal number 37 in binary, how many binary digits are required?
A)
5
done
clear
B)
6
done
clear
C)
7
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 6)
A junction diode has a resistance of \[25\,\Omega \] when forward biased and \[2500\,\,\Omega \]when reverse biased. The current in the diode, for the arrangement shown will be
A)
\[\frac{1}{15}A\]
done
clear
B)
\[\frac{1}{7}A\]
done
clear
C)
\[\frac{1}{25}A\]
done
clear
D)
\[\frac{1}{180}A\]
done
clear
View Answer play_arrow
question_answer 7) If the electron in a hydrogen atom jumps from an orbit with level \[{{n}_{1}}=2\]to an orbit with level \[{{n}_{2}}=1\]the emitted radiation has a wavelength given by
A)
\[\lambda =\frac{5}{3R}\]
done
clear
B)
\[\lambda =\frac{4}{3R}\]
done
clear
C)
\[\lambda =\frac{R}{4}\]
done
clear
D)
\[\lambda =\frac{3R}{4}\]
done
clear
View Answer play_arrow
question_answer 8) What is the particle X in the following nuclear reaction\[_{4}^{9}Be+_{2}^{4}He\xrightarrow{{}}_{6}^{12}C+X\]
A)
Electron
done
clear
B)
Proton
done
clear
C)
Photon
done
clear
D)
Neutron
done
clear
View Answer play_arrow
question_answer 9) An alternating current of rms value 10 A is passed through a \[12\,\Omega \]resistor. The maximum potential difference across the resistor is
A)
20 V
done
clear
B)
90 V
done
clear
C)
169.68 V
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 10) Which of the following relation represents Biot -Savarfs law?
A)
\[\overrightarrow{dB}=\frac{{{\mu }_{0}}}{4\pi }\frac{\overrightarrow{dl}\times \overrightarrow{r}}{r}\]
done
clear
B)
\[\overrightarrow{dB}=\frac{{{\mu }_{0}}}{4\pi }\frac{\overrightarrow{dl}\times \hat{r}}{{{r}^{3}}}\]
done
clear
C)
\[\overrightarrow{dB}=\frac{{{\mu }_{0}}}{4\pi }\frac{\overrightarrow{dl}\times \vec{r}}{{{r}^{3}}}\]
done
clear
D)
\[\overrightarrow{dB}=\frac{{{\mu }_{0}}}{4\pi }\frac{\overrightarrow{dl}\times \vec{r}}{{{r}^{4}}}\]
done
clear
View Answer play_arrow
question_answer 11) \[\overset{\to }{\mathop{\text{A}}}\,\text{ and }\overset{\to }{\mathop{\text{B}}}\,\] are two vectors given by \[\overset{\to }{\mathop{\text{A}}}\,\text{=2}\overset{\hat{\ }}{\mathop{\text{i}}}\,\text{+3}\overset{\hat{\ }}{\mathop{\text{j}}}\,\text{ and }\overset{\to }{\mathop{\text{B}}}\,=\,\overset{\hat{\ }}{\mathop{\text{i}}}\,\text{+}\overset{\hat{\ }}{\mathop{\text{j}}}\,\] The magnitude of the component of \[\overset{\to }{\mathop{\text{A}}}\,\] along \[\overset{\to }{\mathop{\text{B}}}\,\] is
A)
\[\frac{5}{\sqrt{2}}\]
done
clear
B)
\[\frac{3}{\sqrt{2}}\]
done
clear
C)
\[\frac{7}{\sqrt{2}}\]
done
clear
D)
\[\frac{1}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 12) Given \[\vec{C}=\vec{A}\times \vec{B}\]and \[\vec{D}=\vec{B}\times \vec{A}.\]What is the angle between \[\vec{C}\] and \[\vec{D}\]?
A)
\[{{30}^{o}}\]
done
clear
B)
\[{{60}^{o}}\]
done
clear
C)
\[{{90}^{o}}\]
done
clear
D)
\[\text{18}{{\text{0}}^{\text{o}}}\]
done
clear
View Answer play_arrow
question_answer 13) The acceleration a (in\[\text{m}{{\text{s}}^{2}}\]) of a body, starting from rest varies with time t (in s) following the equation \[a=3t+4.\]The velocity of the body at time t = 2 s will be
A)
\[10\,m{{s}^{-1}}\]
done
clear
B)
\[18\,m{{s}^{-1}}\]
done
clear
C)
\[14\,m{{s}^{-1}}\]
done
clear
D)
\[26\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
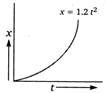
question_answer 14)
Figure below shows the distance-time graph of the motion of a car. It follows from the graph that the car is
A)
at rest
done
clear
B)
in uniform motion
done
clear
C)
in non-uniform acceleration
done
clear
D)
uniformly accelerated
done
clear
View Answer play_arrow
question_answer 15) Two particles have masses m and 4m and their kinetic energies are in the ratio 2:1. What is the ratio of their linear momenta?
A)
\[\frac{1}{\sqrt{2}}\]
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
\[\frac{1}{4}\]
done
clear
D)
\[\frac{1}{16}\]
done
clear
View Answer play_arrow
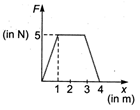
question_answer 16)
The force F acting on a particle moving in a straight line is shown below. What is the work done by the force on the particle in the 1st metre of the trajectory?
A)
5 J
done
clear
B)
10 J
done
clear
C)
15 J
done
clear
D)
2.5 J
done
clear
View Answer play_arrow
question_answer 17) If the kinetic energy of a body changes by 20%, then its momentum would change by
A)
20%
done
clear
B)
24%
done
clear
C)
40%
done
clear
D)
44% (e) None of the above
done
clear
View Answer play_arrow
question_answer 18) A bullet is fired with a velocity \[u\] making an angle of \[\text{6}{{\text{0}}^{\text{o}}}\] with the horizontal plane. The horizontal component of the velocity of the bullet when it reaches the maximum height is
A)
\[u\]
done
clear
B)
0
done
clear
C)
\[\frac{\sqrt{3u}}{2}\]
done
clear
D)
\[\frac{u}{2}\]
done
clear
View Answer play_arrow
question_answer 19) A particle is projected at \[\text{6}{{\text{0}}^{\text{o}}}\] to the horizontal with a kinetic energy KE. The kinetic energy at the highest point is
A)
KE
done
clear
B)
Zero
done
clear
C)
\[\frac{KE}{4}\]
done
clear
D)
\[\frac{KE}{2}\]
done
clear
View Answer play_arrow
question_answer 20) The Poissons ratio of a material is 0.5. If a force is applied to a wire of this material, there is a decrease in the cross-sectional area by 4%. The percentage increase in the length is
A)
1%
done
clear
B)
2%
done
clear
C)
2.5%
done
clear
D)
4%
done
clear
View Answer play_arrow
question_answer 21) Two spheres of equal masses but radii \[{{r}_{1}}\]and \[{{r}_{2}}\]are allowed to fall in a liquid of infinite column. The ratio of their terminal velocities is
A)
1
done
clear
B)
\[{{r}_{1}}:{{r}_{2}}\]
done
clear
C)
\[{{r}_{2}}:{{r}_{1}}\]
done
clear
D)
\[\sqrt{{{r}_{1}}}:\sqrt{{{r}_{2}}}\] (e) None of the above
done
clear
View Answer play_arrow
question_answer 22) Two massless springs of force constants \[{{k}_{1}}\]and \[{{k}_{2}}\]are joined end to end. The resultant force constant k of the system is
A)
\[k=\frac{{{k}_{1}}+{{k}_{2}}}{{{k}_{1}}{{k}_{2}}}\]
done
clear
B)
\[k=\frac{{{k}_{1}}-{{k}_{2}}}{{{k}_{1}}{{k}_{2}}}\]
done
clear
C)
\[k=\frac{{{k}_{1}}{{k}_{2}}}{{{k}_{1}}+{{k}_{2}}}\]
done
clear
D)
\[k=\frac{{{k}_{1}}{{k}_{2}}}{{{k}_{1}}-{{k}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 23) A spring of force constant k is cut into two equal halves. The force constant of each half is
A)
\[\frac{k}{\sqrt{2}}\]
done
clear
B)
k
done
clear
C)
\[\frac{k}{2}\]
done
clear
D)
2k
done
clear
View Answer play_arrow
question_answer 24) Two rods of equal length and diameter have thermal conductivities 3 and 4 units respectively. If they are joined in series, the thermal conductivity of the combination would be
A)
3.43
done
clear
B)
3.5
done
clear
C)
3.4
done
clear
D)
3.34
done
clear
View Answer play_arrow
question_answer 25) 19 g of water at \[\text{30}{{\,}^{\text{o}}}\text{C}\]and 5 g of ice at \[-\text{20}{{\,}^{\text{o}}}\text{C}\]are mixed together in a calorimeter. What is the final temperature of the mixture? (Given specific heat of \[\text{ice}\,\text{=}\,\text{0}\text{.5}\,\text{cal}\,{{\text{g}}^{-1}}{{({{\,}^{o}}C)}^{-1}}\]and latent heat of fusion of \[\text{ice = 80}\,\text{cal }{{\text{g}}^{-1}}\])
A)
\[\text{0}{{\,}^{\text{o}}}\text{C}\]
done
clear
B)
\[-\text{5}{{\,}^{\text{o}}}\text{C}\]
done
clear
C)
\[\text{5}{{\,}^{\text{o}}}\text{C}\]
done
clear
D)
\[10{{\,}^{\text{o}}}\text{C}\]
done
clear
View Answer play_arrow
question_answer 26) It is difficult to cook rice in an open vessel by boiling it at high altitudes because of
A)
low boiling point and high pressure
done
clear
B)
high boiling point and low pressure
done
clear
C)
low boiling point and low pressure
done
clear
D)
high boiling point and high pressure
done
clear
View Answer play_arrow
question_answer 27) The height of a waterfall is 50 m. If \[g=\text{9}\text{.8}\,\text{m}{{\text{s}}^{-2}}\] the difference between the temperature at the top and the bottom of the waterfall is
A)
\[\text{1}\text{.17}{{\,}^{\text{o}}}\text{C}\]
done
clear
B)
\[\text{2}\text{.17}{{\,}^{\text{o}}}\text{C}\]
done
clear
C)
\[\text{0}\text{.117}{{\,}^{\text{o}}}\text{C}\]
done
clear
D)
\[\text{1}\text{.43}{{\,}^{\text{o}}}\text{C}\]
done
clear
View Answer play_arrow
question_answer 28) The distance between an object and a divergent lens is m times the focal length of the lens. The linear magnification produced by the lens is
A)
\[m\]
done
clear
B)
\[\frac{1}{m}\]
done
clear
C)
\[m+1\]
done
clear
D)
\[\frac{1}{m+1}\]
done
clear
View Answer play_arrow
question_answer 29) A 2.0 cm object is placed 15 cm in front of a concave mirror of focal length 10 cm. What is the size and nature of the image?
A)
4 cm, real
done
clear
B)
4 cm, virtual
done
clear
C)
1.0 cm, real
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 30) A beam of monochromatic blue light of wavelength \[\text{4200}\,\overset{\text{o}}{\mathop{\text{A}}}\,\] in air travels in water of refractive index 4/3. Its wavelength in water will be
A)
\[4200\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[5800\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[4150\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[3150\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 31) Two identical light waves, propagating in the same direction, have a phase difference\[\delta \]. After they superpose the intensity of the resulting wave will be proportional to
A)
\[\cos \delta \]
done
clear
B)
\[\cos \left( \frac{\delta }{2} \right)\]
done
clear
C)
\[{{\cos }^{2}}\left( \frac{\delta }{2} \right)\]
done
clear
D)
\[{{\cos }^{2}}\delta \]
done
clear
View Answer play_arrow
question_answer 32) The equation of state for n moles of an ideal gas is \[pV=nRT,\] where R is a constant. The SI unit for R is
A)
\[J{{K}^{-1}}\] per molecule
done
clear
B)
\[J{{K}^{-1}}\,mo{{l}^{-1}}\]
done
clear
C)
\[JK{{g}^{-1}}{{K}^{-1}}\]
done
clear
D)
\[J{{K}^{-1}}{{g}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 33) At a certain place, the horizontal component of earths magnetic field is \[\sqrt{3}\] times the vertical component. The angle of dip at that place is
A)
\[\text{3}{{\text{0}}^{\text{o}}}\]
done
clear
B)
\[\text{6}{{\text{0}}^{\text{o}}}\]
done
clear
C)
\[{{45}^{\text{o}}}\]
done
clear
D)
\[\text{9}{{\text{0}}^{\text{o}}}\]
done
clear
View Answer play_arrow
question_answer 34) The number of electron in 2 coulomb of charge is
A)
\[5\times {{10}^{29}}\]
done
clear
B)
\[12.5\times {{10}^{18}}\]
done
clear
C)
\[1.6\times {{10}^{19}}\]
done
clear
D)
\[9\times {{10}^{11}}\]
done
clear
View Answer play_arrow
question_answer 35) The current flowing through a wire depends on time as\[I=3{{t}^{2}}+2t+5.\] The charge flowing through the cross-section of the wire in time from \[t=0\] to \[t=2\,s\] is
A)
22 C
done
clear
B)
20 C
done
clear
C)
18 C
done
clear
D)
5 C
done
clear
View Answer play_arrow
question_answer 36) If the charge on a capacitor is increased by 2 coulomb, the energy stored in it increases by 21%. The original charge on the capacitor is
A)
10C
done
clear
B)
20C
done
clear
C)
30C
done
clear
D)
40C
done
clear
View Answer play_arrow
question_answer 37) The work done in carrying a charge Q once around a circle of radius r about a charge q at the centre is
A)
\[\frac{qQ}{4\pi {{\varepsilon }_{0}}r}\]
done
clear
B)
\[\frac{qQ}{4\pi {{\varepsilon }_{0}}}\frac{1}{\pi r}\]
done
clear
C)
\[\frac{qQ}{4\pi {{\varepsilon }_{0}}}\left( \frac{1}{2\pi r} \right)\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 38) Four capacitors of equal capacitance have an equivalent capacitance \[{{\text{C}}_{\text{1}}}\] when connected in series and an equivalent capacitance\[{{\text{C}}_{2}}\] when connected in parallel. The ratio \[\frac{{{C}_{1}}}{{{C}_{2}}}\] is
A)
1/4
done
clear
B)
1/16
done
clear
C)
1/8
done
clear
D)
1/12
done
clear
View Answer play_arrow
question_answer 39) Magnetic field intensity H at the centre of a circular loop of radius r carrying current I emu is
A)
\[\frac{r}{I}\] oersted
done
clear
B)
\[\frac{2\pi I}{r}\] oersted
done
clear
C)
\[\frac{I}{2\pi r}\] oersted
done
clear
D)
\[\frac{2\pi r}{I}\] oersted
done
clear
View Answer play_arrow
question_answer 40) Which of the following materials is the best conductor of electricity?
A)
Platinum
done
clear
B)
Gold
done
clear
C)
Silicon
done
clear
D)
Copper
done
clear
View Answer play_arrow
question_answer 41) 1 mole of photon, each of frequency \[2500{{s}^{-1}},\] would have approximately a total energy of
A)
\[1\,erg\]
done
clear
B)
\[1\,J\]
done
clear
C)
\[1\,eV\]
done
clear
D)
\[1\,MeV\]
done
clear
View Answer play_arrow
question_answer 42) If \[{{n}_{t}},\]number of radio atoms are present at time t, the following expression will be a constant
A)
\[\frac{{{n}_{t}}}{t}\]
done
clear
B)
\[\ln \frac{{{n}_{t}}}{t}\]
done
clear
C)
\[\frac{d}{dt}(\ln \,nt)\]
done
clear
D)
\[t.{{n}_{t}}\]
done
clear
View Answer play_arrow
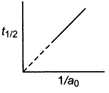
question_answer 43) The following graph shows how \[{{t}_{1/2}}\] (half-life) of a reactant, R changes with the initial reactant concentration \[{{a}_{0}}.\] The order of the reaction will be
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 44)
The second law of thermodynamics says that in a cyclic process
A)
work cannot be converted into heat
done
clear
B)
heat cannot be converted into work
done
clear
C)
work cannot be completely converted into heat
done
clear
D)
heat cannot be completely converted into work
done
clear
View Answer play_arrow
question_answer 45) The equilibrium constant (K) of a reaction may be written as
A)
\[K={{e}^{-\Delta G/RT}}\]
done
clear
B)
\[K={{e}^{-\Delta {{G}^{o}}/RT}}\]
done
clear
C)
\[K={{e}^{-\Delta H/RT}}\]
done
clear
D)
\[K={{e}^{-\Delta {{H }^{o}}/RT}}\]
done
clear
View Answer play_arrow
question_answer 46) For the reaction \[S{{O}_{2}}+\frac{1}{2}{{O}_{2}}\rightleftharpoons S{{O}_{3}},\]if we write\[{{K}_{p}}={{K}_{c}}{{(RT)}^{x}},\] then \[x\]becomes
A)
\[-1\]
done
clear
B)
\[-\frac{1}{2}\]
done
clear
C)
\[\frac{1}{2}\]
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 47) If it is assumed that \[_{\text{92}}^{\text{235}}\text{U}\]decays only by emitting \[\alpha -\]and \[\beta -\]particles, the possible product of the decay is
A)
\[_{89}^{225}Ac\]
done
clear
B)
\[_{89}^{227}Ac\]
done
clear
C)
\[_{89}^{230}Ac\]
done
clear
D)
\[_{89}^{231}Ac\]
done
clear
View Answer play_arrow
question_answer 48) The time taken for 10% completion of a first order reaction is 20 min. Then, for 19% completion, the reaction will take
A)
40 min
done
clear
B)
60 min
done
clear
C)
30 min
done
clear
D)
50 min
done
clear
View Answer play_arrow
question_answer 49) Which of the following will decrease the pH of a 50 mL solution of 0.01 M HCl?
A)
Addition of 5 mL of 1 M HCl
done
clear
B)
Addition of 50 mL of 0.01 M HCl
done
clear
C)
Addition of 50 mL of 0.002 M HCl
done
clear
D)
Addition of Mg
done
clear
View Answer play_arrow
question_answer 50) Equal volumes of molar hydrochloric acid and sulphuric acid are neutralized by dilute NaOH solution and x kcal and y kcal of heat are liberated respectively. Which of the following is true?
A)
\[x=y\]
done
clear
B)
\[x=\frac{y}{2}\]
done
clear
C)
\[~x=2y\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 51) Hybridisation of central atom in \[N{{F}_{3}}\] is
A)
\[s{{p}^{3}}\]
done
clear
B)
\[sp\]
done
clear
C)
\[s{{p}^{2}}\]
done
clear
D)
\[ds{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 52) Of the following compounds, the most acidic is
A)
\[A{{s}_{2}}{{O}_{3}}\]
done
clear
B)
\[{{P}_{2}}{{O}_{5}}\]
done
clear
C)
\[S{{b}_{2}}{{O}_{3}}\]
done
clear
D)
\[B{{i}_{2}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 53) The half-life of a radioactive element is 10 h. How much will be left after 4 h in 1 g atom sample?
A)
\[~45.6\text{ }\times \text{ }{{10}^{23}}\text{ atom}\]
done
clear
B)
\[4.56\text{ }\times \text{ }{{10}^{23}}\text{ atom}\]
done
clear
C)
\[4.56\text{ }\times \text{ }{{10}^{21}}\text{ atom}\]
done
clear
D)
\[4.56\text{ }\times \,\,{{10}^{20}}\text{ atom}\]
done
clear
View Answer play_arrow
question_answer 54) For the Paschen series the values of \[{{n}_{1}}\]and\[{{n}_{2}}\] in the expression\[\Delta E=Rhc\left( \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right)\]are
A)
\[{{n}_{1}}=1,{{n}_{2}}=2,3,4,...\]
done
clear
B)
\[{{n}_{1}}=2,{{n}_{2}}=3,4,5,...\]
done
clear
C)
\[{{n}_{1}}=3,{{n}_{2}}=4,5,6,...\]
done
clear
D)
\[{{n}_{1}}=4,{{n}_{2}}=5,6,7,...\]
done
clear
View Answer play_arrow
question_answer 55) Under which of the following conditions is the relation \[\Delta H=\Delta E+P\Delta V\]valid for a closed system?
A)
Constant pressure
done
clear
B)
Constant temperature
done
clear
C)
Constant temperature and pressure
done
clear
D)
Constant temperature, pressure and composition
done
clear
View Answer play_arrow
question_answer 56) An organic compound made of C, H and N contains 20% nitrogen. Its molecular weight is
A)
70
done
clear
B)
140
done
clear
C)
100
done
clear
D)
65
done
clear
View Answer play_arrow
question_answer 57) In Cu-ammonia complex, the state of hybridisation of \[C{{u}^{2+}}\]is
A)
\[s{{p}^{3}}\]
done
clear
B)
\[{{d}^{3}}s\]
done
clear
C)
\[s{{p}^{2}}f\]
done
clear
D)
\[ds{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 58) The reaction that takes place when \[\text{C}{{\text{l}}_{\text{2}}}\]gas is passed through cone. NaOH solution is
A)
oxidation
done
clear
B)
reduction
done
clear
C)
displacement
done
clear
D)
disproportionation
done
clear
View Answer play_arrow
question_answer 59) Electron is an alloy of
A)
Mg and Zn
done
clear
B)
Fe and Mg
done
clear
C)
Ni and Zn
done
clear
D)
Al and Zn
done
clear
View Answer play_arrow
question_answer 60) Blackened oil painting can be restored into original form by the action of
A)
\[\text{C}{{\text{l}}_{2}}\]
done
clear
B)
\[Ba{{O}_{2}}\]
done
clear
C)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
D)
\[Mn{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 61) Of the following acids the one which has the capability to form complex compound and also possesses oxidising and reducing properties is
A)
\[HN{{O}_{3}}\]
done
clear
B)
\[HN{{O}_{2}}\]
done
clear
C)
\[HCOOH\]
done
clear
D)
\[HCN\]
done
clear
View Answer play_arrow
question_answer 62) Atoms in a \[{{P}_{4}}\] molecule of white phosphorus are arranged regularly in the following way
A)
at the corners of a cube
done
clear
B)
at the corners of an octahedron
done
clear
C)
at the corners of a tetrahedron
done
clear
D)
at the centre and corners of a tetrahedron
done
clear
View Answer play_arrow
question_answer 63) Which of the following statements is not correct?
A)
Silicon is extensively used as a semiconductor
done
clear
B)
Carborundum is SiC
done
clear
C)
Silicon occurs in free state in nature
done
clear
D)
Mica contains the element silicon
done
clear
View Answer play_arrow
question_answer 64) In aluminium extraction by the Bayer process, alumina is extracted from bauxite by sodium hydroxide at high temperatures and pressures \[A{{l}_{2}}{{O}_{3}}(s)+2O{{H}^{-}}(aq)\to 2A{{l}_{2}}O_{2}^{-}(aq)\] \[+{{H}_{2}}O(l)\] Solid impurities such as \[\text{F}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}}\]and \[\text{Si}{{\text{O}}_{2}}\]are removed and then \[Al(OH)_{4}^{-}\]is precipitated \[2Al(OH)_{4}^{-}\xrightarrow{{}}A{{l}_{2}}{{O}_{3}}.3{{H}_{2}}O(s)+2O{{H}^{-}}(aq).\]In the industrial world
A)
carbon dioxide is added to precipitate the alumina
done
clear
B)
temperature and pressure are dropped and the super saturated solution seeded
done
clear
C)
both and are practiced
done
clear
D)
the water is evaporated
done
clear
View Answer play_arrow
question_answer 65) The addition of HBr to 2-pentene gives
A)
2-bromopentane only
done
clear
B)
3-bromopentane only
done
clear
C)
2-bromopentane and 3-bromopentane
done
clear
D)
1-bromopentane and 3-bromopentane
done
clear
View Answer play_arrow
question_answer 66) Ethylene can be separated from acetylene by passing the mixture through
A)
fuming \[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
pyrogallol
done
clear
C)
ammoniacal\[C{{u}_{2}}C{{l}_{2}}\]
done
clear
D)
charcoal powder
done
clear
View Answer play_arrow
question_answer 67) Reaction of R OH with R MgX produces
A)
\[RH\]
done
clear
B)
\[RH\]
done
clear
C)
\[R-R\]
done
clear
D)
\[R-R\]
done
clear
View Answer play_arrow
question_answer 68) In the compound \[HC=C-\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{C}}\,}}\,=C{{H}_{2}}\]the hybridisation of C-2 and C-3 carbons are respectively
A)
\[s{{p}^{3}}\] and\[s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{2}}\]and \[s{{p}^{3}}\]
done
clear
C)
\[s{{p}^{2}}\]and \[~sp\]
done
clear
D)
\[s{{p}^{3}}\]and sp
done
clear
View Answer play_arrow
question_answer 69)
The two structures written below represent
A)
pair of diastereomers
done
clear
B)
pair of enantiomers
done
clear
C)
same molecule
done
clear
D)
both are optically inactive
done
clear
View Answer play_arrow
question_answer 70) Which of the following carbocations will be most stable?
A)
\[P{{h}_{3}}{{C}^{+}}\]
done
clear
B)
\[C{{H}_{3}}-\overset{+}{\mathop{C}}\,{{H}_{2}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}\overset{+}{\mathop{C}}\,H\]
done
clear
D)
\[C{{H}_{2}}=CH-\overset{+}{\mathop{C}}\,{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 71) Which statement is incorrect?
A)
Phenol is a weak acid
done
clear
B)
Phenol is an aromatic compound
done
clear
C)
Phenol liberates \[C{{O}_{2}}\]from \[N{{a}_{2}}C{{O}_{3}}\] solution
done
clear
D)
Phenol is soluble in NaOH
done
clear
View Answer play_arrow
question_answer 72) In which of the following reactions new carbon-carbon bond is not formed?
A)
Cannizaro reaction
done
clear
B)
Wurtz reaction
done
clear
C)
Aldol condensation
done
clear
D)
Friedel-Craft reaction
done
clear
View Answer play_arrow
question_answer 73) A compound is formed by substitution of two chlorine for two hydrogens in propane. The number of possible isomeric compounds is
A)
4
done
clear
B)
3
done
clear
C)
5
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 74) Which one of the following is called a carbylamine?
A)
\[RCN\]
done
clear
B)
\[RCON{{H}_{2}}\]
done
clear
C)
\[RCH=NH\]
done
clear
D)
\[~RNC\]
done
clear
View Answer play_arrow
question_answer 75) For making distinction between 2-pentanone and 3-pentanone, the reagent to be employed is
A)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}/{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[Zn-Hg/HCl\]
done
clear
C)
\[Se{{O}_{2}}\]
done
clear
D)
Iodine/NaOH
done
clear
View Answer play_arrow
question_answer 76) Which one of the following formulae does not represent an organic compound?
A)
\[{{C}_{4}}{{H}_{10}}{{O}_{4}}\]
done
clear
B)
\[{{C}_{4}}{{H}_{8}}{{O}_{4}}\]
done
clear
C)
\[{{C}_{4}}{{H}_{7}}Cl{{O}_{4}}\]
done
clear
D)
\[{{C}_{4}}{{H}_{9}}{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 77) The catalyst used for olefin polymerisation is
A)
Ziegler-Natta catalyst
done
clear
B)
Wilkinson catalyst
done
clear
C)
Raney nickel catalyst
done
clear
D)
Merrifield resin
done
clear
View Answer play_arrow
question_answer 78) The oxidant which is used as an antiseptic is
A)
\[KBr{{O}_{3}}\]
done
clear
B)
\[KMn{{O}_{4}}\]
done
clear
C)
\[Cr{{O}_{3}}\]
done
clear
D)
\[KN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 79) Which of the following contributes to the double helical structure of DNA?
A)
Hydrogen bond
done
clear
B)
Covalentbond
done
clear
C)
Disulphide bond
done
clear
D)
van der Waals force
done
clear
View Answer play_arrow
question_answer 80) The monomer used to produce orlon is
A)
\[C{{H}_{2}}=CHF\]
done
clear
B)
\[C{{H}_{2}}=CC{{l}_{2}}\]
done
clear
C)
\[C{{H}_{2}}=CHCl\]
done
clear
D)
\[C{{H}_{2}}=CH-CN\]
done
clear
View Answer play_arrow
question_answer 81) The length of DNA having 23 base pair is
A)
\[78\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[78.4\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[74.8\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[78.2\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 82) Which Ig is produced in primary immune response?
A)
IgA
done
clear
B)
IgE
done
clear
C)
IgG
done
clear
D)
IgM
done
clear
View Answer play_arrow
question_answer 83) The average diameter of red blood corpuscles of man is
A)
7.2 \[\mu \]m
done
clear
B)
8.1 \[\mu \]m
done
clear
C)
9.2 \[\mu \]m
done
clear
D)
10.3 \[\mu \]m
done
clear
View Answer play_arrow
question_answer 84) FAD is electron acceptor during oxidation of which of the following?
A)
a- ketoglutarate\[\to \]Succinyl Co- A
done
clear
B)
Succinic acid \[\to \] Fumaric acid
done
clear
C)
Succinyl Co- A \[\to \] Succinic acid
done
clear
D)
Fumaric acid \[\to \] Malic acid
done
clear
View Answer play_arrow
question_answer 85) The chemical nature of hormones secreted by a and \[\alpha \,and\,\beta \]cells of pancreas is
A)
glycolipid
done
clear
B)
glycoprotein
done
clear
C)
steroid
done
clear
D)
polypeptide
done
clear
View Answer play_arrow
question_answer 86) The genetic material of rabies virus is
A)
double stranded RNA
done
clear
B)
single stranded RNA
done
clear
C)
double stranded DNA
done
clear
D)
ssDNA
done
clear
View Answer play_arrow
question_answer 87) T-lymphocyte is produced in
A)
bone marrow
done
clear
B)
spleen
done
clear
C)
pancreas
done
clear
D)
thymus
done
clear
View Answer play_arrow
question_answer 88) How many ATP molecules are obtained from fermentation of 1 molecule of glucose?
A)
2
done
clear
B)
4
done
clear
C)
3
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 89) Number of nitrogenous bases in a codon is
A)
3
done
clear
B)
2
done
clear
C)
1
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 90) A character, which is expressed in a hybrid is called
A)
dominant
done
clear
B)
recessive
done
clear
C)
co- dominant
done
clear
D)
epistatic
done
clear
View Answer play_arrow
question_answer 91) In which stage of cell division chromosomes are most condensed?
A)
Prophase
done
clear
B)
Metaphase
done
clear
C)
Anaphase
done
clear
D)
Telophase
done
clear
View Answer play_arrow
question_answer 92) Which of the following is correct?
A)
Haemophilic-Y- chromosome
done
clear
B)
Downs syndrome-21st chromosome
done
clear
C)
Sickle cell anaemia- X-chromosome
done
clear
D)
Parkinsons disease-X and Y- chromosome
done
clear
View Answer play_arrow
question_answer 93) Genetically engineered bacteria are being employed for production of
A)
thyroxin
done
clear
B)
human insulin
done
clear
C)
cortisol
done
clear
D)
epinephrine
done
clear
View Answer play_arrow
question_answer 94) Scientific name of sunflower is
A)
Hibiscus rosa- sinensis
done
clear
B)
Solanum nigrum
done
clear
C)
Oryza sativa
done
clear
D)
Helianthus annuus
done
clear
View Answer play_arrow
question_answer 95) By which of the following methods, new and better varieties of plants can be formed?
A)
Selection
done
clear
B)
Grafting
done
clear
C)
Hybridization
done
clear
D)
Hybridization followed by selection
done
clear
View Answer play_arrow
question_answer 96) Which one is product of aerobic respiration?
A)
Malic acid
done
clear
B)
Ethyl alcohol
done
clear
C)
Lactic acid
done
clear
D)
Pyruvic acid
done
clear
View Answer play_arrow
question_answer 97) \[C{{O}_{2}}\]acceptor in \[{{C}_{3}}\]cycle is
A)
OAA
done
clear
B)
RuBP
done
clear
C)
PEP
done
clear
D)
Malic acid
done
clear
View Answer play_arrow
question_answer 98) Virus was discovered by whom?
A)
Stanley
done
clear
B)
Ivanowski
done
clear
C)
Herelle
done
clear
D)
Beijerinck
done
clear
View Answer play_arrow
question_answer 99) Electron microscope is based on the principle of
A)
electromagnetic theory
done
clear
B)
resolution of glass lenses
done
clear
C)
magnification of glass lenses
done
clear
D)
refraction of light
done
clear
View Answer play_arrow
question_answer 100) Citric acid cycle is the alternate name of which of the following?
A)
HMP shunt
done
clear
B)
Glycolysis
done
clear
C)
TCA cycle
done
clear
D)
Calvin cycle
done
clear
View Answer play_arrow
question_answer 101) Vascular tissue in higher plants develop from which of the following?
A)
Procambium
done
clear
B)
Protoderm
done
clear
C)
Periblem
done
clear
D)
Cortex
done
clear
View Answer play_arrow
question_answer 102) Which element is cause of itai \[\frac{1}{N}\] desh itai disease?
A)
Hg
done
clear
B)
Pb
done
clear
C)
Cd
done
clear
D)
As
done
clear
View Answer play_arrow
question_answer 103) Chromosomes can be stained with one of the following chemicals
A)
acetocarmine
done
clear
B)
safranine
done
clear
C)
light green
done
clear
D)
eosin
done
clear
View Answer play_arrow
question_answer 104) Which one of the following is the American poultry breed?
A)
Australop
done
clear
B)
Minorica
done
clear
C)
Assel
done
clear
D)
Rhod Island Red
done
clear
View Answer play_arrow
question_answer 105) Which part of the human brain is largest?
A)
Cerebellum
done
clear
B)
Thalamus
done
clear
C)
Cerebrum
done
clear
D)
Medulla
done
clear
View Answer play_arrow
question_answer 106) When the other floral parts are arranged at the base of the gynoecium, the flower is called
A)
hypogynous flower
done
clear
B)
perigynous flower
done
clear
C)
epigynous flower
done
clear
D)
agynous flower
done
clear
View Answer play_arrow
question_answer 107) In a CAM plant, the concentration of organic acid
A)
increases during the day
done
clear
B)
decreases or increases during the day
done
clear
C)
increases during night
done
clear
D)
decreases during any time
done
clear
View Answer play_arrow
question_answer 108) Protein coat of virus is known as
A)
capsid
done
clear
B)
virion
done
clear
C)
viroid
done
clear
D)
bacterial wall
done
clear
View Answer play_arrow
question_answer 109) Net yield of ATP molecules in aerobic respiration during Krebs cycle per glucose molecule is
A)
2 ATP molecules
done
clear
B)
8 ATP molecules
done
clear
C)
36 ATP molecules
done
clear
D)
38 ATP molecules
done
clear
View Answer play_arrow
question_answer 110) Feedback inhibition of enzymes is affected by which of the following?
A)
Enzyme
done
clear
B)
Substrate
done
clear
C)
End products
done
clear
D)
Intermediate end products
done
clear
View Answer play_arrow
question_answer 111) The discovery of gibberellins is related with one of the following
A)
blast disease of rice
done
clear
B)
rust disease of wheat
done
clear
C)
bakane disease of rice
done
clear
D)
early blight disease of potato
done
clear
View Answer play_arrow
question_answer 112) Omithophily refers to the pollination by which of the following?
A)
insects
done
clear
B)
birds
done
clear
C)
snails
done
clear
D)
air
done
clear
View Answer play_arrow
question_answer 113) Which of the following is an example of man-made ecosystem?
A)
Herbarium
done
clear
B)
Aquarium
done
clear
C)
Tissue culture
done
clear
D)
Forest
done
clear
View Answer play_arrow
question_answer 114) Respiratory enzymes are present in the following organelle
A)
peroxysome
done
clear
B)
chloroplast
done
clear
C)
mitochondrion
done
clear
D)
lysosome
done
clear
View Answer play_arrow
question_answer 115) Pellagra is caused due to deficiency of the vitamin
A)
thiamine
done
clear
B)
niacin
done
clear
C)
pyridoxin
done
clear
D)
biotin
done
clear
View Answer play_arrow
question_answer 116) Which one of the following leucocytes transforms into macrophages?
A)
Eosinophil
done
clear
B)
Basophil
done
clear
C)
Monocyte
done
clear
D)
Lymphocyte
done
clear
View Answer play_arrow
question_answer 117) Mention the Incubation Period of Plasmodium vivax
A)
10-14 days
done
clear
B)
20-25 days
done
clear
C)
30 days
done
clear
D)
45 days
done
clear
View Answer play_arrow
question_answer 118) The specific region of hypothalamus, responsible for physiological sweat secretion, is
A)
para- ventricular nucleus
done
clear
B)
supra- optic nucleus
done
clear
C)
median eminence
done
clear
D)
pars distalis
done
clear
View Answer play_arrow
question_answer 119) The duration of cardiac cycle is
A)
0.8 s
done
clear
B)
0.8 \[\mu \]s
done
clear
C)
0.08 s
done
clear
D)
0.008 s
done
clear
View Answer play_arrow
question_answer 120) The intensity levels of whispering noise is
A)
10-15 dB
done
clear
B)
20-40 dB
done
clear
C)
45-50 dB
done
clear
D)
50-55 dB
done
clear
View Answer play_arrow
question_answer 121) The wild-life protection act was introduced in
A)
1972
done
clear
B)
1981
done
clear
C)
1986
done
clear
D)
1991
done
clear
View Answer play_arrow
question_answer 122) In honey, the percentage of maltose and other sugar is
A)
9.2
done
clear
B)
8.81
done
clear
C)
10.5
done
clear
D)
11.2
done
clear
View Answer play_arrow
question_answer 123) Identify the correct type of food chain Dead animal\[\to \]Blow fly maggots\[\to \]Common frog\[\to \] Snake
A)
Grazing food chain
done
clear
B)
Detrital food chain
done
clear
C)
Decomposer food chain
done
clear
D)
Predator food chain
done
clear
View Answer play_arrow
question_answer 124) Which is not applicable to the biological species concept?
A)
Hybridization
done
clear
B)
Natural population
done
clear
C)
Reproductive isolation
done
clear
D)
Gene pool
done
clear
View Answer play_arrow
question_answer 125) DNA sequences that code for protein are known as
A)
introns
done
clear
B)
exons
done
clear
C)
control regions
done
clear
D)
intervening sequences
done
clear
View Answer play_arrow
question_answer 126) Which one of the following is a systematic insecticide?
A)
Malathion
done
clear
B)
Parathion
done
clear
C)
Endrin
done
clear
D)
Furadan
done
clear
View Answer play_arrow
question_answer 127) The resolving power of a compound microscope will increase with
A)
decrease in wavelength of light and increase in numerical aperture
done
clear
B)
increase in wavelength of light and decrease in numerical aperture
done
clear
C)
increase in both wavelength of light and numerical aperture
done
clear
D)
decrease in both wavelength of light and numerical aperture
done
clear
View Answer play_arrow
question_answer 128) Osteomalacia is a disease caused by the deficiency of
A)
calciferol
done
clear
B)
retinol
done
clear
C)
tocopherol
done
clear
D)
phylloquinone
done
clear
View Answer play_arrow
question_answer 129) Which is the correct sequence of arrangement of types of WBC in decreasing order in terms of number per \[m{{m}^{3}}\] of human blood?
A)
Eosinophils > Basophils > Neutrophils
done
clear
B)
asophils > Eosinophils > Neutrophils
done
clear
C)
Neutrophils > Eosinophils > Basophils
done
clear
D)
Eosinophils > Neutrophils > Basophils
done
clear
View Answer play_arrow
question_answer 130) Cells in Go-phase of cell cycle
A)
exit cell cycle
done
clear
B)
enter cell cycle
done
clear
C)
suspend cell cycle
done
clear
D)
terminate cell cycle
done
clear
View Answer play_arrow
question_answer 131) Choose the correct non-protein amino add
A)
hydroxyproline
done
clear
B)
hydroxylysine
done
clear
C)
cystine
done
clear
D)
y- amino butyric add
done
clear
View Answer play_arrow
question_answer 132) Seedless banana is
A)
parthenocarpic fruit
done
clear
B)
multiple fruit
done
clear
C)
drupe fruit
done
clear
D)
rue fruit
done
clear
View Answer play_arrow
question_answer 133) The major site of protein breakdown to form free amino acids is in the
A)
kidney
done
clear
B)
spleen
done
clear
C)
liver
done
clear
D)
bone- marrow
done
clear
View Answer play_arrow
question_answer 134) Collagen is a
A)
phosphoprotein
done
clear
B)
globulin
done
clear
C)
derived protein
done
clear
D)
scleroprotein
done
clear
View Answer play_arrow
question_answer 135) The Repeating Unit of glycogen is
A)
fructose
done
clear
B)
mannose
done
clear
C)
glucose
done
clear
D)
galactose
done
clear
View Answer play_arrow
question_answer 136) Grahams law is correlated with
A)
diffusion
done
clear
B)
osmoregulation
done
clear
C)
osmosis
done
clear
D)
absorption
done
clear
View Answer play_arrow
question_answer 137) Which of the following does not act as a neurotransmitter?
A)
Acetylcholine
done
clear
B)
Glutamic add
done
clear
C)
Epinephrine
done
clear
D)
Tyrosine
done
clear
View Answer play_arrow
question_answer 138) The generation of excitation-contraction coupling involves all the following events except
A)
generation of end- plate potential
done
clear
B)
release of calcium from troponin
done
clear
C)
formation of cross-linkages between actin and myosin
done
clear
D)
hydrolysis of ATP to ADP
done
clear
View Answer play_arrow
question_answer 139) In AIDS, HIV kills
A)
Antibody molecule
done
clear
B)
T- helper cell
done
clear
C)
Bone- marrow cells
done
clear
D)
T- cytotoxic cell
done
clear
View Answer play_arrow
question_answer 140) Generally artificial pace-maker consists of one battery made up of
A)
nickel
done
clear
B)
dry cadmium
done
clear
C)
photo sensitive material
done
clear
D)
lithium
done
clear
View Answer play_arrow
question_answer 141) Goitre can occur as a consequence of all the following except
A)
iodine deficiency
done
clear
B)
pituitary adenoma
done
clear
C)
Graves disease
done
clear
D)
excessive intake of exogenous thyroxin
done
clear
View Answer play_arrow
question_answer 142) Pernicious anaemia results due to deficiency of
A)
vitamin- \[{{B}_{1}}\]
done
clear
B)
vitamin- A
done
clear
C)
vitamin- \[{{B}_{12}}\]
done
clear
D)
iron
done
clear
View Answer play_arrow
question_answer 143) Which of the following substances yield less than 4 kcal / mol when its phosphate bond is hydrolysed?
A)
Creatine phosphate
done
clear
B)
ADP
done
clear
C)
Glucose- 6- phosphate
done
clear
D)
ATP
done
clear
View Answer play_arrow
question_answer 144) The genetic deficiency of ADH- receptor leads to
A)
diabetes mellitus
done
clear
B)
glycosuria
done
clear
C)
diabetes insipidus
done
clear
D)
nephrogenic diabetes
done
clear
View Answer play_arrow
question_answer 145) Out of A-T, G-C pairing, bases of DNA may exist in alternate valency state owing to arrangement called
A)
tautomerisational mutation
done
clear
B)
analogue substitution
done
clear
C)
point mutation
done
clear
D)
frameshift mutation
done
clear
View Answer play_arrow
question_answer 146) Cellular totipotency was first demonstrated by
A)
F C Steward
done
clear
B)
Robert Hooke
done
clear
C)
T Schwann
done
clear
D)
A v Leeuwenhoek
done
clear
View Answer play_arrow
question_answer 147) Molecular scissors, which cut DNA at specific site is
A)
pectinase
done
clear
B)
polymerase
done
clear
C)
restriction endonuclease
done
clear
D)
ligase
done
clear
View Answer play_arrow
question_answer 148) \[S{{O}_{2}}\]pollution is indicated by
A)
Desmodium (grasses)
done
clear
B)
Sphagnum (mosses)
done
clear
C)
Usnea (lichens)
done
clear
D)
Cucurbita (climbers)
done
clear
View Answer play_arrow
question_answer 149) Sporopollenin is chemically
A)
homopolysaccharide
done
clear
B)
fatty substance
done
clear
C)
protein
done
clear
D)
heteropolysaccharide
done
clear
View Answer play_arrow
question_answer 150) During replication of DNA, Okazaki fragments are formed in the direction of
A)
3\[\to \] 5
done
clear
B)
5 \[\to \] 3
done
clear
C)
5\[\to \] 5
done
clear
D)
3\[\to \] 3
done
clear
View Answer play_arrow
question_answer 151) The chemical nature of chromatin is as follows
A)
nucleic acids
done
clear
B)
nucleic acid and histone proteins
done
clear
C)
nucleic acids, histone and non- histone proteins
done
clear
D)
nucleic acids and non- histone proteins
done
clear
View Answer play_arrow
question_answer 152) Choose the minor carp from the following
A)
Cyprinus carpio
done
clear
B)
Anguilla sp
done
clear
C)
Labeo bata
done
clear
D)
Ctenopharyngodon idella
done
clear
View Answer play_arrow
question_answer 153) The scientific name of Asian tiger mosquito
A)
Aedes aegypti
done
clear
B)
Aedes albopictus
done
clear
C)
Aedes taeniorhynchus
done
clear
D)
Aedes albolineatus
done
clear
View Answer play_arrow
question_answer 154) The size of filtration slits of glomerulus
A)
10 nm
done
clear
B)
15 run
done
clear
C)
20 nm
done
clear
D)
25 nm
done
clear
View Answer play_arrow
question_answer 155) Ornithorhynchus is an example of
A)
dinosaur
done
clear
B)
monotreme mammal
done
clear
C)
marsupial mammal
done
clear
D)
eutherian mammal
done
clear
View Answer play_arrow
question_answer 156) Scirpophage incertulus is an example of
A)
monophagus pest
done
clear
B)
diphagus pest
done
clear
C)
oligophagus pest
done
clear
D)
polyphagus pest
done
clear
View Answer play_arrow
question_answer 157) Which one of the following ancestors of man first time showed bipedal movement?
A)
Australopithecus
done
clear
B)
Cro- magnon
done
clear
C)
Java ape man
done
clear
D)
Peking man
done
clear
View Answer play_arrow
question_answer 158) Trophic levels in ecosystem is formed by
A)
only bacteria
done
clear
B)
only plants
done
clear
C)
only herbivores
done
clear
D)
organisms linked in food chain
done
clear
View Answer play_arrow
question_answer 159) The life span of honey bee drone is
A)
3-4 months
done
clear
B)
1-2 months
done
clear
C)
6-7 months
done
clear
D)
10-12 months
done
clear
View Answer play_arrow
question_answer 160) Name of a gaseous plant hormone is
A)
IAA
done
clear
B)
gibberellin
done
clear
C)
ethylene
done
clear
D)
abscisic acid
done
clear
View Answer play_arrow