Rutherford's Atomic Model
Category : JEE Main & Advanced
After \[\alpha -\]particles scattering experiment, following conclusions were made by Rutherford as regard as atomic structure :
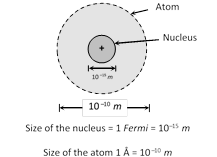
(1) Most of the mass (at least 99.95%) and all of the charge of an atom concentrated in a very small region is called atomic nucleus.
(2) Nucleus is positively charged and it's size is of the order of \[{{10}^{-15}}\,m\approx 1\] Fermi. The nucleus occupies only about \[{{10}^{-12}}\] of the total volume of the atom or less.
(3) In an atom there is maximum empty space and the electrons revolve around the nucleus in the same way as the planets revolve around the sun.
You need to login to perform this action.
You will be redirected in
3 sec
