Failure of Rutherford's Model
Category : JEE Main & Advanced
(1) Stability of atom : It could not explain stability of atom because according to classical electrodynamics theory an accelerated charged particle should continuously radiate energy. Thus an electron moving in an circular path around the nucleus should also radiate energy and thus move into smaller and smaller orbits of gradually decreasing radius and it should ultimately fall into nucleus.
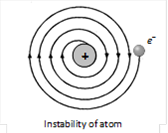
(2) According to this model the spectrum of atom must be continuous where as practically it is a line spectrum.
(3) It did not explain the distribution of electrons outside the nucleus.
You need to login to perform this action.
You will be redirected in
3 sec
