Answer:
(i)
Ambident ligand Some ligands can be coordinated to the metal or metal ion
through either of the sides. They are called ambident ligands.
Examples
(a) ![]() (b) CN
(a) If the
nitrite ion is attached through N-atom
(b) CN
(a) If the
nitrite ion is attached through N-atom ![]() it is
nitro
it is
nitro ![]() ; if it
is attached through 0-atom. (?ONO), then it is nitrito
; if it
is attached through 0-atom. (?ONO), then it is nitrito
![]()
![]() pent
amine-N-nitro cobalt (III) ion ?N? indicates that ligand joined to metal ion through
N-side.
(b) Cyano
?CN if jointed to metal through carbon.
Isocyano
(?NC) if jointed to metal through nitrogen.
[1]
(ii)
Denticity of ligand
pent
amine-N-nitro cobalt (III) ion ?N? indicates that ligand joined to metal ion through
N-side.
(b) Cyano
?CN if jointed to metal through carbon.
Isocyano
(?NC) if jointed to metal through nitrogen.
[1]
(ii)
Denticity of ligand ![]() donate
one pair of electrons in complex formation and are called unidentate ligands.
donate
one pair of electrons in complex formation and are called unidentate ligands.
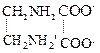 etc.
donate two electron pairs in complex formation and are called bidentate.
When a di-or
polydentate ligand uses its two or more donar atoms to bind a single metal ion,
it is said to be a chelate ligand. The number of such ligating groups is
called denticity of the ligands. Such complexes are called chelate complexes.
They are more stable as compared to complexes containing unidentate ligand.
etc.
donate two electron pairs in complex formation and are called bidentate.
When a di-or
polydentate ligand uses its two or more donar atoms to bind a single metal ion,
it is said to be a chelate ligand. The number of such ligating groups is
called denticity of the ligands. Such complexes are called chelate complexes.
They are more stable as compared to complexes containing unidentate ligand.
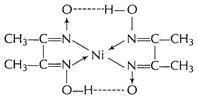 Nickel
dimethyl glyoximate chelate [1]
(iii)
Octahedral field splitting in an octahedral field Five d-orbitals (
Nickel
dimethyl glyoximate chelate [1]
(iii)
Octahedral field splitting in an octahedral field Five d-orbitals (![]() and
and![]() )
are of equal
energy and thus are degenerate.
The
)
are of equal
energy and thus are degenerate.
The ![]() and
and ![]() orbitals
are directed along a set of mutually perpendicular x, y and z-axis. As a group,
these orbitals are called e, orbitals.
Thee
orbitals
are directed along a set of mutually perpendicular x, y and z-axis. As a group,
these orbitals are called e, orbitals.
Thee![]() and
and ![]() orbitals,
I ie between the axis and collectively called
orbitals,
I ie between the axis and collectively called ![]() orbitals.
The ligand
donor atoms approach the metal ion along the axes to from octahedral complexes.
Crystal field theory proposes that the approach of the six donor atoms along
the axes sets up an electric field (crystal field).
Electrons on
the ligand repel electrons in eg orbitals on the metal ion more
strongly than they repel those in
orbitals.
The ligand
donor atoms approach the metal ion along the axes to from octahedral complexes.
Crystal field theory proposes that the approach of the six donor atoms along
the axes sets up an electric field (crystal field).
Electrons on
the ligand repel electrons in eg orbitals on the metal ion more
strongly than they repel those in ![]() orbitals.
This removes the degeneracy of the set of d-orbitals and splits them into two
sets, the eg set at higher energy and the
orbitals.
This removes the degeneracy of the set of d-orbitals and splits them into two
sets, the eg set at higher energy and the ![]() set at
lower energy.
set at
lower energy.
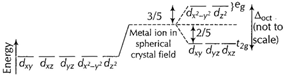 Free metal
ion (into crystal field) The energy separation between the two sets is called
Free metal
ion (into crystal field) The energy separation between the two sets is called ![]() or
or![]() proportional
to the crystal field of the strength of the ligands.
The d-electrons
on a metal ion occupy the
proportional
to the crystal field of the strength of the ligands.
The d-electrons
on a metal ion occupy the ![]() set in preference
to the higher energy, eg set. Electrons that occupy the eg
orbitals are strongly repelled by the relatively close approach of ligands.
Electron in eg set tends to destabilise octahedral complexes.
set in preference
to the higher energy, eg set. Electrons that occupy the eg
orbitals are strongly repelled by the relatively close approach of ligands.
Electron in eg set tends to destabilise octahedral complexes.
You need to login to perform this action.
You will be redirected in
3 sec
