Answer:
(i)
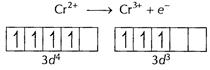 When
When ![]() is
oxidised there is no effect on the stability. Thus
is
oxidised there is no effect on the stability. Thus ![]() is a
reducing agent.
is a
reducing agent.
![]() When
When ![]() is
reduced to
is
reduced to ![]() stability
increases. Thus
stability
increases. Thus ![]() is
an oxidising agent. [1]
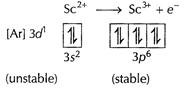
(ii) We can
consider the case of Sc (21)
is
an oxidising agent. [1]
(ii) We can
consider the case of Sc (21)
![]()
![]()
![]() is
unstable and is easily oxidised to
is
unstable and is easily oxidised to ![]() to
attain stable configuration of the inert gas
to
attain stable configuration of the inert gas ![]() Thus, 3d1
configuration is very unstable in ion.
Thus, 3d1
configuration is very unstable in ion.
 [1]
(iii)
[1]
(iii)
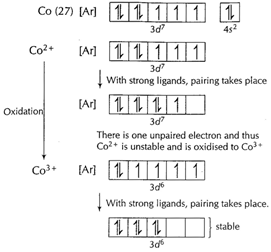 Thus
Thus ![]() is
oxidised to
is
oxidised to ![]() when
complexing takes place.
when
complexing takes place.
You need to login to perform this action.
You will be redirected in
3 sec
