Environmental Chemistry
Category : UPSC
ENVIRONMENTAL CHEMISTRY
CHEMISTRY AND MANKIND
SOME IMPORTANT MOLECULES OF LIFE
Carbohydrate
The chemicals used by the body may be divided into two categories;
Carbohydrates are the main energy sources for the human body. Chemically, carbohydrates are organic molecules in which carbon, hydrogen, and oxygen bond together in the ratio:\[{{C}_{X}}{{({{H}_{2}}O)}_{Y}}\], where X and Y are whole numbers.
Animals obtain carbohydrates by eating foods like potatoes, rice, breads, and so on. These carbohydrates are manufactured by plants during the process of photosynthesis. Plants harvest energy from sunlight to run the reaction just described in reverse:
\[6C{{O}_{2}}+6{{H}_{2}}O+energy\,(from\,\,sunlight)\xrightarrow[{}]{{}}{{C}_{6}}{{H}_{12}}{{O}_{6}}+6{{O}_{2}}\]
There are two types of carbohydrates, the simple sugars and those that are made of long chains of sugars - the complex carbohydrates.
Simple Sugars
All carbohydrates are made up of units of sugar (also called saccharide units). Carbohydrates that contain only one sugar unit (mono saccharides) or two sugar units (disaccharides) are referred to as simple sugars. Simple sugars are sweet in taste and are broken down quickly in the body to release energy. Two of the most common mono saccharides are glucose and fructose. Glucose is the primary form of sugar stored in the human body for energy. Fructose is the main sugar found in most fruits. Both glucose and fructose have the same chemical formula (\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\]); however, they have different structures. Disaccharides have two sugar units bonded together. For example, common table sugar is sucrose, a disaccharide that consists of a glucose unit bonded to a fructose unit.
Sweetening power of common sugars:
Fructose> Invert sugar> Sucrose> Glucose> Maltose> Lactose
Complex Carbohydrates
Complex carbohydrates are polymers of the simple sugars. In other words, the complex carbohydrates are long chains of simple sugar units bonded together. Therefore the complex carbohydrates can also be referred to as polysaccharides. Starch is an example of complex carbohydrate.
Handy Facts
Both starch and glucogen are polymers of glucose; however, starch is a long, straight chain of glucose units, wheras glycogen is a branched chain of glucose units. Another important polysaccharide is cellulose. Cellulose is yet a third polymer of the monosaccharide glucose. Cellulose differs from starch and glycogen in terms of extra stability. Cellulose, also known as plant fibre, cannot be digested by human beings, therefore cellulose passes through the digested tract without being absorbed into the body. Cellulose fibre is essential in the diet because it helps exercise the digestive track and keep it clean and healthy.
AMINO ACIDS AND PROTEINS
Amino Acids
Amino acids play central roles both as building blocks of proteins and as intermediate in metabolism. There twenty amino acids which are present in the protein. Humans can produce 10 of the 20 amino acids. The others must be supplied by food.
Structure of Amino Acids
Proteins
Proteins (also known as polypeptide) are organic compounds made of amino acids arranged in a linear chain and folded into a globular form. The amino acids in a polymer are joined together by the peptide bonds between the carboxyl and amino groups of adjacent amino acid residues.
Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cyloskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signalling immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animal’s diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.
Peptides
Peptides are short polymers formed from the linking in a defined order, of an amino acid residue and the next is called an amide bond or a peptide bond.
Peptides play a crucial role in fundamental physiological and biochemical functions of life. For decades now, peptide research is a continuously growing field of science.
Peptide is a molecule formed by joining two or more amino acids. When the number of amino acids is less than about 50, these molecules formed by peptides while larger sequences are referred to as proteins.
The amino acids are coupled by a peptide bond, a special linkage in which the nitrogen atom of one amino acid binds to the carboxyl carbon atom of another.
Peptides (proteins) are present in every living cell and possess a variety of biochemical activities. They appear as enzymes, hormones, antibiotics, receptors, etc.
Handy Facts
When proteins are heated above body temperature or when they are subjected to unusual acid or base conditions or treated with special reagents called denaturants, they lose some or all of their tertiary and secondary structure. Proteins in this state no longer exhibit normal biological activities and are called denatured proteins.
OIL AND FATS
Fats consist of a wide group of compounds that are generally soluble in organic solvents and largely insoluble in water. Chemically, fats are generally triesters of glycerol and fatty acids. Fats may be either solid or liquid at room temperature, depending on their structure and composition.
Hydrogenation of Oils
Unsaturated vegetable fats and oils can be transformed through partial or complete hydrogenation into fats and oils of higher melting point. The hydrogenation process involves “sparging” the oil at high temperature and pressure with hydrogen in the presence of a catalyst, typically a powdered nickel compound. As each double-bond is broken, two hydrogen atoms each form single bonds with the two carbon atoms. The elimination of double-bonds by adding hydrogen atoms is called saturation; as the degree of saturation increases, the oil progresses towards being fully hydrogenated, An oil may be hydrogenated to increase resistance to rancidity (oxidation) or to change its physical characteristics. As the degree of saturation increases, the oil’s viscosity and melting point increase.
![]()
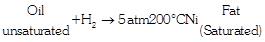
CHEMISTRY
HORMONES
Hormones are the substances produced by ductless or endocrine glands and poured directly into the blood stream.
Effects of Hormone
Hormones have the following effects on the body:
A hormone may also regulate the production and release of other hormones. Hormone signals control the internal environment of the body through homeostasis.
Enzyme
Enzymes are the biocatalysts of life. They are defined as biocatalysts synthesized by living cells. They are simple or conjugate protein and specific in action. At present about 300 enzymes are recognized and classified into six classes by International Union of Biochemistry (IUB). They are: Oxidoreductases, Transferases, Hydrolases, Lyases, Isomerases and Ligases.
Functions of Enzymes
Science in Action
Deficiency of some enzymes causes disease like albinism in individuals. Albinism is caused due to the deficiency of enzyme tyrosinase. Phenyl ketonuria is caused due to the deficiency of enzyme phenyl alanine hydroxylase.
Vitamins
Vitamins are organic compounds required in the diet in small amounts to perform specific biological functions for normal maintenance of optimum growth and health of the organism. Vitamins are designated by alphabets A, B, C, D, etc., some of them are further named as sub-groups, e. g., \[{{B}_{1}}\], \[{{B}_{2}}\], \[{{B}_{6}}\], \[{{B}_{12}}\], etc.
Vitamins are classified into two groups:
Some important vitamins, their sources and diseases caused by their deficiency are listed in the following table:
|
Sr. No. |
Name of Vitamins |
Source |
Deficiency Diseases |
|
1. |
Vitamin A (Retinol) |
Cod, liver oil, carrots, egg, butter and milk |
Xerophthalmia (hardening of cornea of eye) Night blindness |
|
2. |
Vitamin B 1 (Thiamine) |
Seeds, whole grains, Pulses, nuts |
Beri-beri (loss of appetite, retarded growth) |
|
3. |
Vitamin B 2 (Riboflavin) |
Milk, egg white, liver, Kidney |
Cheilosis (fissuring at comers of mouth and lips), digestive disorders and burning sensation of the skin |
|
4. |
Vitamin B 5 (Nicotinamid) |
Barley, liver maize, wheat |
Pellagra (skin pigmentation) degeneration of spinal cord) |
|
5. |
Vitamin B 6 (Pyridoxine) |
Yeast, milk, egg yolk, rice, cereals and grams |
Anaemia |
|
6. |
Vitamin B 12 (Cyanocobalamine) |
Meat, fish, egg and curd |
Pernicious anaemia (RBC deficient in hemoglobin) |
|
7. |
Vitamin C (Ascorbic acid) |
Citrus fruits, amla and green leafy vegetables |
Scurvy (bleeding gums) |
|
8. |
Vitamin D (Calciferol) |
Exposure to sunlight, fish and egg yolk |
Rickets (bone deformities in children) and osteomalacia (soft bones and joint pain in adults) |
|
9. |
Vitamin E (Tocoferol) |
Vegetable oils like wheat germ oil, cotton seed oil, sunflower oil, etc. |
Increased fragility of RBCs and muscular weakness, Antifertility |
|
10. |
Vitamin K (phyllo-quinone) |
Green leafy vegetables |
Increased blood clotting time |
MAN-MADE MOLECULES
Polymers
A polymer may by defined as a high molecular weight compound termed by the combination of a large number of one or more types of small molecules of low molecular weight. The small unit (or units) of which the polymer is made is known as monomer (or monomers). Many polymeric substances occur in nature such as cellulose, starch, rubber, proteins, and resins.
The synthetic polymers are manufactured generally from the small units by the process known as polymerisation. Polymerisation may be defined as a chemical combination of a number of similar or different molecules to form a single large molecule.
Addition Polymerisation
This involves the self-addition of n-unsaturated molecules of one or two monomers without loss of any small molecule to form a single giant molecule, e.g., propylene polymerises to polypropylene.
Some of the polymers are obtained only from one type of monomer, whereas the others are obtained from two different types of monomers, the former polymers are known as photopolymers whereas the latter are known as copolymers. Polypropylene, polyethylene, polyisoprens, etc. are the examples of photopolymers; on the other hand, dacron, nylon, certain vinyl polymers, etc. constitute the examples of' copolymers.
Some Common Example of Addition and Condensation Polymers and their uses:
|
Addition Polymers |
Repeating Unit |
Common Uses |
|
Polyethylene (PE) |
|
Plastic bages, bottles |
|
Polypropylene (PP) |
|
Indoor-outdoor carpets |
|
Polystyrene (PS) |
|
Plastic utensils, insulation |
|
Polyvinyl chloride (PVC) |
|
Shower curtains, tubing |
|
Polyvinylidene chloride (Saran) |
|
Plastic warp |
|
Polytetrafluoroethylene (Teflon) |
|
Non-stick coating |
|
Polyacrylonitrile (Orion) |
|
Yarn, paints |
|
Polymethyl methacrylate (Lucite, Plexiglas) |
|
Windows, bowling balls |
|
Polyvinyl acetate (PVA) |
|
Adhesives, chewing gum |
|
Nylon |
|
Carpeting, clothing |
|
Polyethylene terephthalate |
|
Clothing, plastic bottles |
|
Melamine-formaldehyde resin (Melmac, Formica) |
|
Dishes, countertops. |
Polymers may be divided into two categories
Rayon was originally called artificial silk but now a days it is a name given to artificial fibres derived form cellulose. Rayon can absorb over 90% of its own mass of water and it was not stick to wound.
Natural silk contains nitrogen while artificial silk may not have nitrogen.
Science in Action
Rubber a well-known organic polymer and the only true hydrocarbon polymer found in nature. It is formed by the radical addition of the monomer isoprene. Polymerization can result in either poly-cis-isoprene or poly-trans isoprene-or a mixture of both, depending on reaction conditions. Natural rubber is poly-cis-isoprene, which is extracted from the tree Hevea brasiliensis.
In 1839, the American chemist Charles Goodyear discovered that natural rubber could be cross-linked with sulphur (using zinc oxide as the catalyst) to maintain its elasticity even under external pressure. His process, known as vulcanization, paved the way for many practical and commercial uses of rubber, such as in automobile tire and dentures.
Most synthetic rubbers (called elastomers) are made from petroleum products such as ethylene, propene, and butadiene. For example, chloroprene molecules polymerize readily to form polychloroprene, commonly known as neoprene, which has properties that are comparable or even superior to those of natural rubber.
Dye
A dye can generally be described as a colored substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and may require a mordant to improve the fastness of the dye on the fibre.
The dyes were obtained from animal, vegetable or mineral origin, with no or very little processing.
Classification of dye
Dyes may be classified as discussed below:
Acid dyes: These are water-soluble anionic dyes that are applied to fibres such as silk, wool, nylon and modified aery lie fibres using neutral to acid dye baths.
Basic dyes: These are water-soluble cationic dyes that are mainly applied to acrylic fibres, but find some use for wool and silk. Usually acetic acid is added to the dye bath to help the uptake of the dye onto the fibre. Basic dyes are also used in the coloration of paper.
Direct or substantive dyes: These kind of dyes are obtained when dyeing is normally carried out in a neutral or slightly saline dye bath, at or near boiling point, with the addition of either sodium chloride (NaCl) or sodium sulphate (\[N{{a}_{2}}S{{O}_{4}}\]). Direct dyes are used on cotton, paper, leather, wool, silk and nylon. They are also used as pH indicators and as biological stains.
Mordant dyes: These require a mordant, which improves the fastness of the dye against water, light and perspiration.
Science in Action
The most important mordant dyes are the synthetic mordant dyes, or chrome dyes, used for wool; these comprise some 30% of dyes used for wool, and are especially useful for black and navy shades. The mordents, potassium dichromate, is applied as an after treatment.
Vat dyes: These are essentially insoluble in water and incapable of dyeing fibres directly.
Reactive dyes: These utilize a chromophore attached to a substituent that is capable of directly reacting with the fibre substrate. Reactive dyes are by far the best choice for dyeing cotton and other cellulose fibres at home or in the art studio.
Disperse dyes: These were originally developed for the dyeing of cellulose acetate, and are water insoluble. The dyes are finely ground in the presence of a dispersing agent and sold as a paste, or spray-dried and sold as a powder. Their main use is to dye polyster but they can also be used to dye nylon, cellulose triacetate, and acrylic fibres.
Pigments
Pigments are various organic and inorganic insoluble substances, which are widely used as surface coatings. They are also employed in the ink, plastic, rubber, ceramic, paper and linoleum industries to impart colour. The pigment industry is usually regarded as associated with paints, but in fact it is a separate industry.
Pigments are broadly classified into two types:
Paints
Paints are stable mechanical mixtures of one or more pigments. The main function of the pigments is to impart the desired colour and to protect the paint film from penetrating radiation, such as U.V. rays.
The important varieties of paints are emulsion paints, latex paints, metallic paints, epoxide resin paints, oil paints, water paints or distempers, etc.
Drugs and Medicines
Chemical substances administered to a human body or to an animal, either for treatment of diseases or reduce suffering from pain are called medicines or drugs. The various types of medicinal compounds according to the purpose for which they are used are:
Antiseptics
The chemicals which prevent or check the sepsis of wounds. Examples are: dettol (chloroxylenol + terpeneol), Bithional (added to soaps). Salol, Acriflavin, Savlon, Gention violet, Mercuro Chrome, Salicylic acid, picric acid, resorcinol, phenol, iodoform, boric acid, iodine, methylene blue, potassium permanganate.
Disinfectants
The chemical substances which completely destroy the micro-organisms or stop their growth but are harmful to human tissues are called disinfectants. Examples are: 1.0% phenol,\[S{{O}_{2}}\]etc.
Antipyretics
They lower the body temperature i.e. fever reducing. Examples are: aspirin, para-cetamol, phenacetin, analgin.
Analgesics
They are pain releiving. Examples are: Aspirin and analgin, both are antipyretic and analgesics. Novalgin is most widely used analgesic.
Certain narcotics like Codeine, morphene, pethidine hydrochloride, methadone and heroin etc. are also used as analgesics.
Tranquilizers
Also called psycho-therapeutic drugs, reduce anxiety, induce sleep, and cure mental diseases. Examples are: Barbituric acid and its derivatives, seconal, luminal, Methyldopa and Hydralazine, Equanil.
Sedatives and Hypnotics
They are central nervous system depressants reduce restlessness, emotional tension and induce sleep. Examples are: Phenobarbital, Glute thimide, Valizim etc.
Antianxiety Agents
Examples are: Meprobamate and Diazepam.
Tranquilizers, Sedatives & Hypnotics and Antianxiety agents are central nervous system stimulants.
Anaesthetics
The chemical substances which produce insensibility to the vital functions of all types of cell especially of nervous system temporarily are called anaesthetics. Examples are, General anaesthetics - Chloroform, Fluothane, Local anaesthetics -Cocaine, \[\alpha \]-Eucaine, \[\beta \]-Eucaine.
Narcotics
The chemical substances which act as depressant and analgesic.
Examples: Heroin, Opium and Pethedine.
Antibiotics
The chemical substances produced by micro-organisms that inhibit the growth of bacteria or even destroy them are called antibiotics. Examples: Penicillin is the first antibiotic discovered by Alexander Fleming. It is effective against pneumonia, bronchitis and sore throat etc.
Antimalarials
These are the drugs which cure malaria. Examples are:
Plasmoquin (Plasmochin), Atebrin (Mepacrine), Chloroquine.
Antacids
Antacids are the drugs which neutralize excess acid in the gastric Juices and give relief from acid indigestion. They remove the excess acid and raise the pH to appropriate level in stomach. There are mainly weak bases.
Examples- \[Mg{{(OH)}_{2}},KHC{{O}_{3}}\]
Fertilizer
A chemical fertilizer is defined as any inorganic material of wholly or partially synthetic origin that is added to the soil to sustain plant growth.
Classification of Fertilizers
Based on the availability of nutrients in them chemical fertilizers are divided into four groups:
Science in Action
Long term use of chemical fertilizer causes harms to the eco-system. Some fertilizers are highly acidic, which in turn often increases the acidity of the soil, thereby reducing beneficial organisms and stunting plant growth. By upsetting this natural ecosystem, long-term use of synthetic fertilizer can eventually lead to a chemical imbalance in the recipient plants. Hence chemical fertilizer needs to be applied in moderation, as too much can easily “burn” the plants and sometimes even kill them.
Pesticides
Pesticides are a class of synthetic chemicals used to control the pests of crops. Pests can be an insect, disease or weed or sometimes non - insect pest like rats.
Some important pesticides commonly used in agriculture are:
Handy Facts
Pesticides can cause large scale pollution – as less than 5% of applied pesticide reaches the actual pests and balance 95% enters the eco-system. The pesticides may remain in plant bodies and may accumulate in edible part of crops of pesticides and may persist in soils to cause serious damage to biological balance in the soil. Pesticides can also enter ground water and may act as source of water pollution.
Cement
Portland cement is the basic ingredient of concrete. Concrete is formed when Portland cement creates a paste with water (called hydration) that binds with sand and rock to harden. Bricklayer Joseph Aspdin of Leeds, England first made Portland cement early in the 19th century by burning powdered limestone and clay.
Manufacture of Portland Cement
Portland cement is manufactured by crushing, milling and proportioning the following materials:
The most common way to manufacture Portland cement is through a dry method:
70% of the cement produced in the world is Portland cement.
Safety Matches
The credit for developing friction matches that are presently still in use has been attributed to English chemist, John Walker, in 1826.
Ink
Ink is a colloidal system of fine pigment particles dispersed in a solvent. The pigment may or may not be coloured, and the solvent may be aqueous or organic. We can have two types of inks: Writing inks and Printing inks
Handy Facts
Dyes tend to be preferred over pigments for writing inks because pigments can’t be dispersed minutely enough and tend to clog the pen tip. And water-based dye or pigment systems are still used for markers, highlighters, and roller ball pens.
Gun-powder
Gunpowder is a mixture of three different components:
Handy Facts
Nitro-glycerine was first made in 1847. It is hazardous to make, use and transport. In 1886, Alfred Noel found that nitro-glycerine soaks into diatomaceous earth to give a pasty mixture that can be molded into sticks that don’t detonate so easily. These were called dynamite and Noble started the company Dynamite Noble to manufacture dynamite. He made lots of money through dynamite business and later funded the Nobel prizes.
Soaps and Detergents
Soaps are water-soluble sodium or potassium salts of fatty acids,
The common alkalis used in soap making are sodium hydroxide (NaOH), also called caustic soda; and potassium hydroxide (KOH), also called caustic potash.
Saponification of fats and oils is the most widely used soap making process. Saponification involves heating fats and oils and reacting them with a liquid alkali to produce soap and water (neat soap) plus glycerine.
Cleaning action of soaps and detergents:
Water alone will not remove grease or oil from clothes because oil and grease present in soil repel the water molecules. Both soaps and detergents share a critical chemical property – they are surface-active agents, or surfactants. In other words, they reduce the surface tension of water. Because of reduction of surface tension, water soaks more easily in clothes and removes stains faster.
The carboxylate end of the soap molecule is attracted to water. It is called the hydrophilic (water-loving) end. The hydrocarbon chain is attracted to oil and grease and repelled by water. It is known as the hydrophobic (water-hating) end.
The water-hating end is repelled by water but attracted to the oil in the soil. At the same time, the water-loving end is attracted to the water molecules. These opposing forces loosen the soil and suspend it in the water. Warm or hot water helps dissolve grease and oil in soil. Washing machine agitation or hand rubbing helps pull the soil free.
Fuels
A fuel is a substance that releases energy. Some fuels (for example uranium) release energy from nuclear reactions.
Types of Fossil Fuels
Fossil fuels can be separated into three categories:
Disadvantages: Coal is a very polluting fuel that produces a large amount of unburnt hydrocarbon, particulate, and significant quantities of sulphur dioxide by products.
Factors to consider when choosing a fuel
Energy Value: Energy Value is the heat of combustion of a fuel given per gram of fuel. The higher the energy value, the more energy is released, the better the fuel.
Ignition Temperature: Ignition Temperature is the minimum temperature to which the fuel-oxidizer mixture (or a portion of it) must be heated in order for the combustion reaction to occur.
CHEMISTRY AND THE ENVIRONMENT
The environment consists of various segments such as atmosphere, hydrosphere, lithosphere and biosphere.
Atmosphere: The atmosphere is the protective layer of gases which is surrounding the earth. Its main functions are:
Hydrosphere: Hydrosphere is the part of earth on which all types of water resources exist, viz., oceans, seas, rivers, lakes, glaciers, ice caps, ground water, etc.
Lithosphere: Lithosphere is the part of the earth where all types of minerals, metals, organic matters, rocks, soils, etc. exist. Soil is a part of lithosphere.
Biosphere: The biosphere refers to the sphere of living organisms and their interactions with the environment (viz. atmosphere, hydrosphere and lithosphere). The biosphere is very large and complex and is divided into smaller units called ecosystems.
DAMAGE TO ENVIRONMENT
Environment may get damaged due to several reasons. The damage may be in the small area or may affect a much larger area and its ill-effects may by felt all over the globe. The environmental damages may be broadly classified as:
Regional Environmental Damage
Those environmental damages which affect the living and non-living things locally over a small area are termed as regional environmental damages.
Examples are:
Acid rain
Acid rain, or acid deposition, is a broad term that includes any form on precipitation with acidic components, such as sulphuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms. This may include rain, snow, fog, hail or even dust that is acidic. Acid rain results when sulphur dioxide (\[S{{O}_{2}}\]) and nitrogen oxides (\[N{{O}_{X}}\]) are emitted into the atmosphere and transported by wind and air currents. The \[S{{O}_{2}}\] and\[N{{O}_{X}}\] react with water, oxygen and other chemicals to form sulphuric and nitric acids. These then mix with water and other material before falling to the ground. The major sources of \[S{{O}_{2}}\] and \[N{{O}_{X}}\] in the atmosphere are:
The environmental effects of acid rain include:
Major pollutants of air
|
Pollutants |
Primary sources |
Significant Effects |
|
\[S{{O}_{2}}\] |
Vehicular combustion, fossil burning |
Acid rain, irritation in eyes, premature falling of leaves |
|
\[CO\] and \[C{{O}_{2}}\] |
Vehicular combustion, burning of fuels and hydrocarbons |
Global warming, green-house effect, CO has great affinity for hydrocarbons, hemoglobin and forms carboxy hemoglobin |
|
Smoke, fly ash and soot |
Thermal power stations |
Respiratory diseases. |
|
Lead and mercury |
Auto exhaust from gasoline (petrol), paints, storage batteries. Fossil fuel like coal burning. |
Affects the nervous system and circulatory system causing nerve fuel burning and brain damage |
|
CFCs (Chlorofluorocarbon) |
Refrigerants and aerosol |
Kidney damage and ozone depletion. |
Major pollutants of water
|
Pollutants |
Primary sources |
Significant Effects |
|
Pesticides and insecticides |
Improper use in agriculture like DDT, BHC, mosquito repellants |
Toxic to fishes, predatory birds and mammals |
|
Plastics |
Homes and industries |
Kills fishes and animals. Persists in the environment because of non-bio degrabality. |
|
Chlorine compounds |
Water disinfection with chlorine, paper industries and bleaching powder |
Fatal for plankton (organisms floating on the surface of water), foul taste and odour, can cause Cancer in humans. |
|
Lead paints |
Leaded gasoline |
Toxic to organisms |
|
Mercury |
Natural evaporation and dissolved industrial wastes, fungicides |
Highly toxic to humans |
|
Acids Mine drainage, industrial wastes |
Mine drainage, industrial wastes |
Kills organisms |
|
Sediments |
Natural erosion, run off from factories mining and construction activities, fertilizer and other |
Reduces ability of water to assimilate oxygen. |
Radioactive Pollution
Radioactive Pollution can be defined as the release of radioactive substances or high-energy particles into the air, water, or earth as a result of human activity, either by accident or by design.
The sources of such waste include: (1) nuclear weapon testing or detonation; (2) the nuclear fuel cycle, including the mining separation, and production of nuclear materials for use in nuclear power plants or nuclear bombs; (3) accidental release of radioactive material from nuclear power plants.
Some worst environmental disasters
|
Name of the disaster |
Reason for occurrence |
Effect of the disaster |
|
Bhopal Gas Leak (1983) |
Toxic gases (Methyl iso-cyanide-\[C{{H}_{3}}NC\]) leaked from the Union Carbide (now Dow Chemical) pesticide plant in Bhopal, India |
The harmful fumes spread into the sleeping city and people woke with burning eyes and lungs. Thousands died within days. Years later also, pollutants seeping out of the plant site into groundwater have caused cancer, growth retardation and dizziness. |
|
Tokaimura Nuclear Plant (1999) |
Japan?s worst nuclear accident happened in a facility northeast of Tokyo. |
Two ended up dying, and hundreds were exposed to various levels of radiation. |
|
Minamata disease (1956) |
Industrial poisoning of Minamata Bay in Japan. Large amounts of mercury and other heavy metals were released into the waste water. |
Large amounts of mercury and other heavy metals found their way into the fish and shellfish that comprised a large part of the local diet. Thousands of residents of the Minamata have slowly suffered over the decades and died from a disease-termed as Minamata disease. |
|
The Great Smog of (1952 in London) |
A smog covered London for 5 days in 1952. Cold weather, combined with windless conditions collected airborne pollutants from the use of coal to form a thick layer of smog over the city. |
Thousands died and a hundred thousand fell ill because of a blanket of smog. An estimated 12,000 premature deaths have been attributed to this smog. |
|
Baia Mare Cyanide Spill (2000) |
Cyanide-contaminated water leaked out from a dam, leaking out 100 tonnes of cyanide in to Baia Mare lake in Romania. It has been considered as the second fatal environmental next to Chernobyl in Russia which has been discussed already. |
An incredible amount of fish and aquatic plants were killed and up to 100 people were hospitalized after eating contaminated fish. |
Handy Facts
Dioxin is a general term that describes a group of hundreds of chemicals that are highly persistent in the environment. The most toxin compound is 2, 3, 7, 8– tetrachlorodibenzo-p-dioxin or TCDD. Dioxin and furan are some of the most-toxin compounds.
Dissolved Oxygen (DO)
Oxygen dissolved in water is vital for aquatic life. The optimum value for dissolved oxygen in good quality water is 4-8 mg/L. It is consumed by oxidation of organic matter/ reducing agent etc. present in water. Water which has DO value less than 4 mg/L is termed as polluted and is unfit for human or aquatic animal consumptions.
Chemical Oxygen Demand (COD)
It is an index of the organic content of water, since the most common substance oxidized by the dissolved oxygen in water is organic matter, from a biological origin, such as dead plants etc.
Biological Oxygen Demand (BOD)
The capacity of the organic matter in the sample of natural water to consume oxygen is called its BOD. It is determined experimentally by determining the dissolved oxygen (DO) at the beginning and at the end of a 5-day period in a sealed sample. The BOD gives the measure of oxygen utilized or consumed in the period as a result of oxidation of dissolved organic matter present in the water sample.
Threshold Limit Value (TLV)
This value indicates the permissible level of a toxic pollutant in atmosphere to which a healthy industrial worker can be exposed during an eight-hour day without any adverse effect.
Smog
The word smog is derived from smoke and fog. There are two types of smog: classical and photochemical smog. Classical smog occurs in cool humid climate. It is a mixture of smoke, fog and sulphur dioxide. It is also called reducing smog. Whereas photochemical smog occurs in warm and dry sunny climate. It has high concentration of oxidizing agents and therefore, it is also called as oxidizing smog.
Bio magnification
This refers to increase in concentration of the toxicant at successive trophic levels. This happens because a toxic substance accumulated by an organisation can-not be mitobolised or excreted, and is thus passed on to the next higher trophic level. Bio magnification happens in the aquatic food chain. This is well known for mercury and DDT.
Eutrophication
The process in which nutrient enriched water bodies support a dense plant population, which kills animal life by depriving it of oxygen and results in subsequent loss of biodiversity, is known as Eutrophication.
Science in Action
Photochemical smog mainly contains ozone, nitric oxide, acrolein, formaldehyde and peroxyacetyl nitrate (PAN). These cause serious health problems. In order to stop photochemical smog, catalytic converter are used now-a-days in cars so that release of \[N{{O}_{2}}\]and hydrocarbons are controlled.
You need to login to perform this action.
You will be redirected in
3 sec
