Carnot Engine
Category : JEE Main & Advanced
(1) Carnot designed a theoretical engine which is free from all the defects of a practical engine. This engine cannot be realised in actual practice, however, this can be taken as a standard against which the performance of an actual engine can be judged.
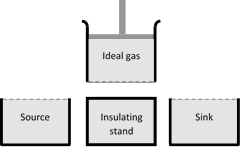
It consists of the following parts
(i) A cylinder with perfectly non-conducting walls and a perfectly conducting base containing a perfect gas as working substance and fitted with a non-conducting frictionless piston
(ii) A source of infinite thermal capacity maintained at constant higher temperature \[{{T}_{1}}\].
(iii) A sink of infinite thermal capacity maintained at constant lower temperature \[{{T}_{2}}\].
(iv) A perfectly non-conducting stand for the cylinder.
(2) Carnot cycle : As the engine works, the working substance of the engine undergoes a cycle known as Carnot cycle. The Carnot cycle consists of the following four strokes
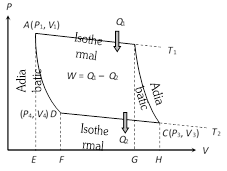
(i) First stroke (Isothermal expansion) (curve AB) :
The cylinder containing ideal gas as working substance allowed to expand slowly at this constant temperature \[{{T}_{1}}\].
Work done = Heat absorbed by the system
\[{{W}_{1}}={{Q}_{1}}=\int_{\,{{V}_{1}}}^{\,{{V}_{2}}}{P\,dV}=R{{T}_{1}}{{\log }_{e}}\left( \frac{{{V}_{2}}}{{{V}_{1}}} \right)=\]Area ABGE
(ii) Second stroke (Adiabatic expansion) (curve BC) :
The cylinder is then placed on the non conducting stand and the gas is allowed to expand adiabatically till the temperature falls from \[{{T}_{1}}\] to \[{{T}_{2}}\].
\[{{W}_{2}}=\int_{\,{{V}_{2}}}^{\,{{V}_{3}}}{P\,dV}=\frac{R}{(\gamma -1)}[{{T}_{1}}-{{T}_{2}}]=\]Area BCHG
(iii) Third stroke (Isothermal compression) (curve CD) :
The cylinder is placed on the sink and the gas is compressed at constant temperature \[{{T}_{2}}\].
Work done = Heat released by the system
\[{{W}_{3}}={{Q}_{2}}=-\int_{\,{{V}_{3}}}^{\,{{V}_{4}}}{\,P\,dV}=-R{{T}_{2}}{{\log }_{e}}\frac{{{V}_{4}}}{{{V}_{3}}}\]
\[=R{{T}_{2}}{{\log }_{e}}\frac{{{V}_{3}}}{{{V}_{4}}}=\text{Area }CDFH\,\]
(iv) Fourth stroke (adiabatic compression) (curve DA) : Finally the cylinder is again placed on non-conducting stand and the compression is continued so that gas returns to its initial stage.
\[{{W}_{4}}=-\int_{\,{{V}_{4}}}^{\,{{V}_{1}}}{P\,dV}=-\frac{R}{\gamma -1}({{T}_{2}}-{{T}_{1}})\]\[=\frac{R}{\gamma -1}({{T}_{1}}-{{T}_{2}})=\text{Area }ADFE\]
(3) Efficiency of Carnot cycle : The efficiency of engine is defined as the ratio of work done to the heat supplied i.e.
\[\eta =\frac{\text{Work done}}{\text{Heat input}}=\frac{W}{{{Q}_{1}}}\]
Net work done during the complete cycle
\[W={{W}_{1}}+{{W}_{2}}+(-{{W}_{3}})+(-{{W}_{4}})\]\[={{W}_{1}}-{{W}_{3}}=\text{Area }ABCD\] [As \[{{W}_{2}}={{W}_{4}}\]]
\[\therefore \] \[\eta =\frac{W}{{{Q}_{1}}}=\frac{{{W}_{1}}-{{W}_{3}}}{{{W}_{1}}}=\frac{{{Q}_{1}}-{{Q}_{2}}}{{{Q}_{1}}}=1-\frac{{{W}_{3}}}{{{W}_{1}}}=1-\frac{{{Q}_{2}}}{{{Q}_{1}}}\]
or \[\eta =1-\frac{R{{T}_{2}}{{\log }_{e}}({{V}_{3}}/{{V}_{4}})}{R{{T}_{1}}{{\log }_{e}}({{V}_{2}}/{{V}_{1}})}\]
Since points B and C lie on same adiabatic curve
\[\therefore \] \[{{T}_{1}}V_{2}^{\gamma -1}={{T}_{2}}V_{3}^{\gamma -1}\] or \[\frac{{{T}_{1}}}{{{T}_{2}}}={{\left( \frac{{{V}_{3}}}{{{V}_{2}}} \right)}^{\gamma -1}}\] ...(i)
Also point D and A lie on the same adiabatic curve
\[\therefore \] \[{{T}_{1}}V_{1}^{\gamma -1}={{T}_{2}}V_{4}^{\gamma -1}\] or \[\frac{{{T}_{1}}}{{{T}_{2}}}={{\left( \frac{{{V}_{4}}}{{{V}_{1}}} \right)}^{\gamma -1}}\] ...(ii)
From (i) and (ii), \[\frac{{{V}_{3}}}{{{V}_{2}}}=\frac{{{V}_{4}}}{{{V}_{1}}}\] or \[\frac{{{V}_{3}}}{{{V}_{4}}}=\frac{{{V}_{2}}}{{{V}_{1}}}\]\[\Rightarrow \]\[{{\log }_{e}}\left( \frac{{{V}_{3}}}{{{V}_{4}}} \right)={{\log }_{e}}\left( \frac{{{V}_{2}}}{{{V}_{1}}} \right)\]
So efficiency of Carnot engine \[\eta =1-\frac{{{T}_{2}}}{{{T}_{1}}}\]
(i) Efficiency of a heat engine depends only on temperatures of source and sink and is independent of all other factors.
(ii) All reversible heat engines working between same temperatures are equally efficient and no heat engine can be more efficient than Carnot engine (as it is ideal).
(iii) As on Kelvin scale, temperature can never be negative (as 0 K is defined as the lowest possible temperature) and \[{{T}_{1}}\] and \[{{T}_{2}}\] are finite, efficiency of a heat engine is always lesser than unity, i.e., whole of heat can never be converted into work which is in accordance with second law.
(4) Carnot theorem : The efficiency of Carnot's heat engine depends only on the temperature of source (T1) and temperature of sink \[({{T}_{2}})\], i.e., \[\eta =1-\frac{{{T}_{2}}}{{{T}_{1}}}\].
Carnot stated that no heat engine working between two given temperatures of source and sink can be more efficient than a perfectly reversible engine (Carnot engine) working between the same two temperatures. Carnot's reversible engine working between two given temperatures is considered to be the most efficient engine. Difference Between Petrol Engine and Diesel Engine
| Petrol engine | Diesel engine |
| Working substance is a mixture of petrol vapour and air. | Working substance in this engine is a mixture of diesel vapour and air. |
| Efficiency is smaller \[(\tilde{\ }47%)\]. | Efficiency is larger \[(\tilde{\ }55%)\]. |
| It works with a spark plug. | It works with an oil plug. |
| It is associated with the risk of explosion, because petrol vapour and air is compressed. So, low compression ratio is kept. | No risk of explosion, because only air is compressed. Hence compression ratio is kept large. |
| Petrol vapour and air is created with spark plug. | Spray of diesel is obtained through the jet. |
You need to login to perform this action.
You will be redirected in
3 sec
