Tri-halides (Chloroform and iodoform)
Category : JEE Main & Advanced
Chloroform or trichloromethane, CHCl3
It is an important trihalogen derivative of methane. It was discovered by Liebig in 1831 and its name chloroform was proposed by Dumas as it gave formic acid on hydrolysis. In the past, it was extensively used as anaesthetic for surgery but now it is rarely used as it causes liver damage.
(1) Preparation
(i) Chloroform is prepared both in the laboratory and on large scale by distilling ethyl alcohol or acetone with bleaching powder and water. The yield is about 40%. The available chlorine of bleaching powder serves both as oxidising as well as chlorinating agent.
\[\underset{\text{Bleaching}\,\text{powder}}{\mathop{CaOC{{l}_{2}}}}\,+{{H}_{2}}O\xrightarrow{{}}Ca{{(OH)}_{2}}+C{{l}_{2}}\]
(a) From alcohol
\[[C{{l}_{2}}+{{H}_{2}}O\xrightarrow{{}}2HCl+O]\]
\[\underset{\text{Ethyl}\,\text{alcohol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,+O\xrightarrow{{}}\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,+{{H}_{2}}O\]
\[\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,+3C{{l}_{2}}\xrightarrow{{}}\underset{\text{Chloral}}{\mathop{CC{{l}_{3}}CHO}}\,+3HCl\]
[So \[C{{l}_{2}}\] acts both as an oxidising and chlorinating agent]
Chloral, thus, formed, is hydrolysed by calcium hydroxide.
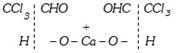
\[\xrightarrow{\text{Hydrolysis}}\underset{\text{Chloroform}}{\mathop{2CHC{{l}_{3}}}}\,+\underset{\text{Calcium}\,\text{formate}}{\mathop{{{(HCOO)}_{2}}Ca}}\,\]
(b) From acetone
\[C{{H}_{3}}-CO-C{{H}_{3}}+3C{{l}_{2}}\xrightarrow{{}}\underset{\text{Trichloro}\,\text{acetone}}{\mathop{CC{{l}_{3}}COC{{H}_{3}}}}\,+3HCl\]
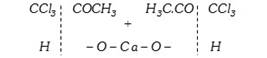
\[\xrightarrow{\text{Hydrolysis}}\underset{\text{Chloroform}}{\mathop{2CHC{{l}_{3}}}}\,+\underset{\text{Calcium}\,\text{acetate}}{\mathop{{{(C{{H}_{3}}COO)}_{2}}Ca}}\,\]
(ii) From carbon tetrachloride : Now-a-days, chloroform is obtained on a large scale by the reduction of carbon tetrachloride with iron fillings and water.
\[CC{{l}_{4}}+2H\xrightarrow{Fe/{{H}_{2}}O}CHC{{l}_{3}}+HCl\]
This chloroform is not pure and used mainly as a solvent.
(iii) Pure Chloroform is obtained by distilling chloral hydrate with concentrated sodium hydroxide solution.
\[\underset{\text{Chloral}\,\text{hydrate}}{\mathop{CC{{l}_{3}}CH{{(OH)}_{2}}}}\,+NaOH\xrightarrow{{}}CHC{{l}_{3}}+HCOONa+{{H}_{2}}O\]
(2) Physical properties
(i) It is a sweet smelling colourless liquid.
(ii) It is heavy liquid. Its density is 1.485. It boils at \[{{61}^{o}}C\].
(iii) It is practically insoluble in water but dissolves in organic solvents such as alcohol, ether, etc.
(iv) It is non-inflammable but its vapours may burn with green flame.
(v) It brings temporary unconsciousness when vapours are inhaled for sufficient time.
(3) Chemical properties
(i) Oxidation :
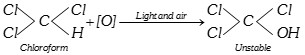
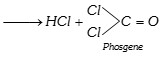
Phosgene is extremely poisonous gas. To use chloroform as an anaesthetic agent, it is necessary to prevent the above reaction. The following two precautions are taken when chloroform is stored.
(a) It is stored in dark blue or brown coloured bottles, which are filled upto the brim.
(b) 1% ethyl alcohol is added. This retards the oxidation and converts the phosgene formed into harmless ethyl carbonate.
\[COC{{l}_{2}}+2{{C}_{2}}{{H}_{5}}OH\xrightarrow{{}}\,\underset{\text{Ethyl carbonate}}{\mathop{{{({{C}_{2}}{{H}_{5}}O)}_{2}}CO}}\,+2HCl\]
(ii) Reduction :
\[CHC{{l}_{3}}+2H\underset{\text{(alc}\text{.)}}{\mathop{\xrightarrow{Zn/HCl}}}\,C{{H}_{2}}C{{l}_{2}}+HCl\]
\[CHC{{l}_{3}}+6H\xrightarrow{Zn/{{H}_{2}}O}C{{H}_{4}}+3HCl\]
(iii) Chlorination :
\[CHC{{l}_{3}}+C{{l}_{2}}\xrightarrow{\text{UV light}}\underset{\text{Carbon tetrachloride}}{\mathop{CC{{l}_{4}}}}\,+HCl\]
(iv) Hydrolysis :
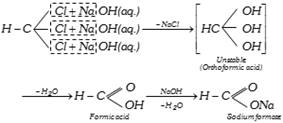
(v) Nitration : The hydrogen of the chloroform is replaced by nitro group when it is treated with concentrated nitric acid. The product formed is chloropicrin or trichloronitro methane or nitro chloroform. It is a liquid, poisonous and used as an insecticide and a war gas.
\[CHC{{l}_{3}}+\underset{\text{Nitric}\,\text{acid}}{\mathop{HON{{O}_{2}}}}\,\xrightarrow{{}}\underset{\text{Chloropicrin}\,\text{(Tear gas)}}{\mathop{CN{{O}_{2}}C{{l}_{3}}}}\,+{{H}_{2}}O\]
(vi) Heating with silver powder :
![]()
(vii) Condensation with acetone : Chloroform condenses with acetone on heating in presence of caustic alkalies. The product formed is a colourless crystalline solid called chloretone and is used as hypnotic in medicine.
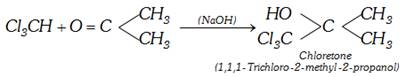
(viii) Reaction with sodium ethoxide :
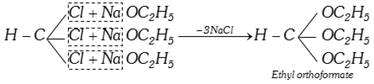
(ix) Reimer-Tiemann reaction :
\[{{C}_{6}}{{H}_{5}}OH+CHC{{l}_{3}}+3NaOH\xrightarrow{65{}^\circ C}\]
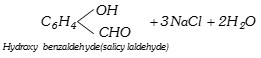
(x) Carbylamine reaction (Isocyanide test) : This reaction is actually the test of primary amines. Chloroform, when heated with primary amine in presence of alcoholic potassium hydroxide forms a derivative called isocyanide which has a very offensive smell.
\[RN{{H}_{2}}+CHC{{l}_{3}}+3KOH(alc.)\xrightarrow{\Delta }\underset{\text{(Alkyl}\,\text{isonitrile)}}{\mathop{\underset{\text{Carbylaminoalkane}}{\mathop{RNC}}\,}}\,+3KCl+3{{H}_{2}}O\]
This reaction is also used for the test of chloroform.
(4) Uses
(i) It is used as a solvent for fats, waxes, rubber, resins, iodine, etc.
(ii) It is used for the preparation of chloretone (a drug) and chloropicrin (Insecticide).
(iii) It is used in laboratory for the test of primary amines, iodides and bromides.
(iv) It can be used as anaesthetic but due to harmful effects it is not used these days for this purpose.
(v) It may be used to prevent putrefaction of organic materials, i.e., in the preservation of anatomical species.
(5) Tests of chloroform
(i) It gives isocyanide test (Carbylamine test).
(ii) It forms silver mirror with Tollen's reagent.
(iii) Pure Chloroform does not give white precipitate with silver nitrate.
Iodoform or tri-iodomethane, CHI3
Iodoform resembles chloroform in the methods of preparation and properties.
(1) Preparation
(i) Laboratory preparation :
From ethanol : \[C{{H}_{3}}C{{H}_{2}}OH+{{I}_{2}}\xrightarrow{{}}\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,+2HI\]
\[C{{H}_{3}}CHO+3{{I}_{2}}\xrightarrow{{}}\underset{Iodal}{\mathop{C{{I}_{3}}CHO}}\,+3HI\]
\[\underset{Tri-iodoacetaldehyde}{\mathop{C{{I}_{3}}CHO}}\,+KOH\xrightarrow{{}}\underset{\text{Iodoform}}{\mathop{CH{{I}_{3}}}}\,+\underset{\text{Pot}\text{. formate}}{\mathop{HCOOK}}\,\]
From Acetone: \[C{{H}_{3}}COC{{H}_{3}}+3{{I}_{2}}\xrightarrow{{}}\underset{\text{Tri}-\text{iodoacetone}}{\mathop{C{{I}_{3}}COC{{H}_{3}}}}\,+3HI\]
\[C{{I}_{3}}COC{{H}_{3}}+KOH\xrightarrow{{}}\underset{\text{Iodoform}}{\mathop{CH{{I}_{3}}}}\,+\underset{\text{Pot}\text{.}\,\text{acetate}}{\mathop{C{{H}_{3}}COOK}}\,\]
Sodium carbonate can be used in place of KOH or NaOH. These reactions are called iodoform reactions.
(ii) Industrial preparation : Iodoform is prepared on large scale by electrolysis of a solution containing ethanol, sodium carbonate and potassium iodide. The iodine set free, combine with ethanol in presence of alkali to form iodoform. The electrolysis carried out in presence of \[C{{O}_{2}}\] and the temperature is maintained at \[60-{{70}^{o}}C\].
![]()
\[K+{{H}_{2}}O\xrightarrow{{}}KOH+\frac{1}{2}{{H}_{2}}\]
KOH is neutralised by \[C{{O}_{2}}\]:
\[{{C}_{2}}{{H}_{5}}OH+4{{I}_{2}}+3N{{a}_{2}}C{{O}_{3}}\xrightarrow{{}}CH{{I}_{3}}\]\[+HCOONa+5NaI+3C{{O}_{2}}+2{{H}_{2}}O\]
(2) Physical properties
(i) It is a yellow crystalline solid.
(ii) It has a pungent characteristic odour.
(iii) It is insoluble in water but soluble in organic solvents such as alcohol, ether, etc.
(iv) It has melting point \[{{119}^{o}}C\]. It is steam volatile.
(3) Chemical Reactions of iodoform
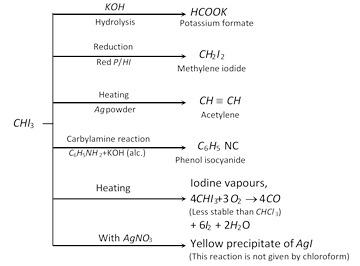
(4) Uses : Iodoform is extensively used as an antiseptic for dressing of wounds; but the antiseptic action is due to the liberation of free iodine and not due to iodoform itself. When it comes in contact with organic matter, iodine is liberated which is responsible for antiseptic properties.
(5) Tests of iodoform
(i) With AgNO3 : \[CH{{I}_{3}}\] gives a yellow precipitate of \[AgI\].
(ii) Carbylamine reaction : \[CH{{I}_{3}}\] on heating with primary amine and alcoholic KOH solution, gives an offensive smell of isocyanide (Carbylamine).
(iii) Iodoform reaction : With \[{{I}_{2}}\] and \[NaOH\] or \[{{I}_{2}}\] and \[N{{a}_{2}}C{{O}_{3}}\], the iodoform test is mainly given by ethyl alcohol \[(C{{H}_{3}}C{{H}_{2}}OH),\]acetaldehyde \[(C{{H}_{3}}\overset{O}{\mathop{-\overset{||}{\mathop{C}}\,-}}\,H),\] \[\alpha -\]methyl ketone or 2-one \[(\overset{O}{\mathop{-\overset{||}{\mathop{C}}\,-}}\,C{{H}_{3}}),\] secondary alcohols or 2-ol \[(-CHOH\cdot C{{H}_{3}})\] and secondary alkyl halide at \[{{C}_{2}}(-CHCIC{{H}_{3}})\]. Also lactic acid (\[C{{H}_{3}}-CHOH-COOH)\], Pyruvic acid \[(C{{H}_{3}}\overset{O}{\mathop{-\overset{||}{\mathop{C}}\,-}}\,COOH)\] and methyl phenyl ketone \[({{C}_{6}}{{H}_{5}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{3}})\] give this test.
You need to login to perform this action.
You will be redirected in
3 sec
