Indicators
Category : 7th Class
Indicators are used to indicate the nature of a substance. Chemical compounds have been categorized into three groups, that is, acid, base and salt, according to their nature. The substance whose nature is acidic (sour in test), called acid and whose nature is basic (bitter in test) is called base. Litmus is the natural indicator and used for the testing of the solution. Litmus paper turns blue to red if the solution is acid, and it turns red to blue if the solution is base. Litmus paper is widely used in the chemical laboratory.
Look at the following picture of Litmus

Acid Base
The natural colour of litmus is purple. Litmus is obtained from a type of tree called lichen. The colour of the natural litmus is converted into red and blue for the application in chemical experiment.
Look at the Following Picture of Lichen (Source of Litmus)

Phenolphthalein is a solution used as an indicator in the chemical laboratory it is a synthetic indicator. Colour of phenolphthalein does not change in the acidic solution, while in the basic solution it turns into pink colour
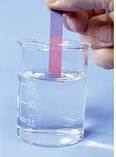
Look at the Following Picture of Phenolphthalein in Indicator

In the above picture, there are three beakers, one has phenolphthalein. If we Put a drop of acid into it, the colour of does not change. But if we put a drop of base into it, the color of indicator turns into pink. Again a drop of acid into the pink colored beaker turns it colorless- Acid cancels the effect of the basic component. The substance whose colour does not change in any solution of indicator is called neutral substance. The ch.na rose is also used as the indicator and called natural indicator Acids turn china rose indicator into magenta (deep pink). Bases turn China rose indicator into green. Another indicator is turmeric paper. In the acidic solution it turns into yellow. In the basic solution, it turns into red. Ph value is used to show the nature of the solution.
| Ph numbers | Colours | Strong/weak Acids/bases | Examples |
| 1 | Red | Strong acid | |
| 2, 3 | Pink | Weak Acid | Lemon juice Stomach juice |
| 4, 5 | Orange | Weak Acid | Lemon juice Stomach juice |
| 6 | Yellow | Weak Acid | Lemon juice Stomach juice |
| 7 | Green | Weak/neutral acid | Milk, Pure water |
| 8, 9, 10, 11 | Light blue | Weak solution | Bicarbonate, ammonia, water |
| 12 | Dark | ||
| 13, 14 | Dark blue | Strong alkali |

![]() Which one of the following completely dissolves in water?
Which one of the following completely dissolves in water?
(a) ![]()
(b) Sand
(c) ![]()
(d) All of these
(e) None of these
Answer: (c)
![]() The litmus is used to show the nature of the substances. Which one of the following changes occurs in the litmus paper in acidic solution?
The litmus is used to show the nature of the substances. Which one of the following changes occurs in the litmus paper in acidic solution?
(a) Colorless
(b) Turns blue to red
(c) Turns red to blue
(e) All of these
(e) None of these
Answer: (b)
You need to login to perform this action.
You will be redirected in
3 sec
