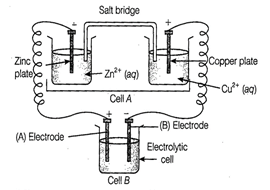
 (i) Cell 'A' has
(i) Cell 'A' has Answer:
(i) Cell 'B' will act as
electrolytic cell due to its lesser value of emf.
The electrode reactions will be
At cathode ![]() At anode
At anode ![]() If cell 'B' has higher emf, it
acts as galvanic cell.
Now it will push electrons into
cell 'A' In this case, the reactions will be
If cell 'B' has higher emf, it
acts as galvanic cell.
Now it will push electrons into
cell 'A' In this case, the reactions will be
![]() (At anode)
(At anode)
![]() (At cathode)
(At cathode)
You need to login to perform this action.
You will be redirected in
3 sec
