Answer:
[Ni(CN)4]2?.
Nickel in this complex ion is in the +2 oxidation state.
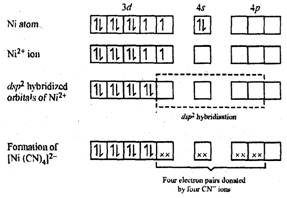 CN? ion
is a strong ligand and cause pairing of unpaired electrons.
dsp2?hybrid
orbitals accommodate four pairs of electrons from four
CN? ion
is a strong ligand and cause pairing of unpaired electrons.
dsp2?hybrid
orbitals accommodate four pairs of electrons from four![]() groups and the
resulting complex is square planar and is diamagnetic since no unpaired
electron is present.
groups and the
resulting complex is square planar and is diamagnetic since no unpaired
electron is present.
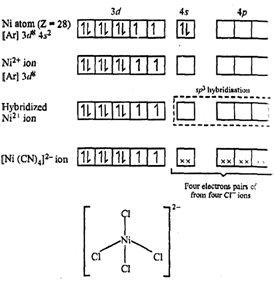
 Tetrahloronickelate
(II) ion.
Nickel in this complex is in the +2 oxidation state. The vacant 4s-orbital and
three 4p-oprbitals hybridise to give four equivalent sp3hybridised
orbitals. Four pairs of electrons, one from each
Tetrahloronickelate
(II) ion.
Nickel in this complex is in the +2 oxidation state. The vacant 4s-orbital and
three 4p-oprbitals hybridise to give four equivalent sp3hybridised
orbitals. Four pairs of electrons, one from each ![]() ion occupy the four
vacant hybrid orbitals so produced. The resulting complex has a tetrahedral
geometry and is paramagnetic because of two unpaired electrons.
ion occupy the four
vacant hybrid orbitals so produced. The resulting complex has a tetrahedral
geometry and is paramagnetic because of two unpaired electrons.
You need to login to perform this action.
You will be redirected in
3 sec
