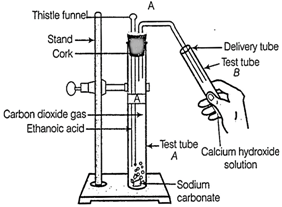
 (a) What
change would you observe in the calcium hydroxide solution taken in tube B?
(b) Write
the reaction involved in test tubes A and B respectively.
(c) If
ethanol is given instead of ethanoic acid, would you expect the same change?
(d) How can
a solution of lime water be prepared in the laboratory?
(a) What
change would you observe in the calcium hydroxide solution taken in tube B?
(b) Write
the reaction involved in test tubes A and B respectively.
(c) If
ethanol is given instead of ethanoic acid, would you expect the same change?
(d) How can
a solution of lime water be prepared in the laboratory?
Answer:
(a) When CO2is
passed through calcium hydroxide solution (taken in test tube B), i.e.,
limewater, it turns milky due to the formation of insoluble calcium carbonate
(CaCO3)
(b) Test tube A
![]()
![]() Test tube
B
Test tube
B
![]() (c) If
ethanol is taken instead of ethanoic acid, no change will occur because ethanol
is a very weak acid and hence cannot decompose
(c) If
ethanol is taken instead of ethanoic acid, no change will occur because ethanol
is a very weak acid and hence cannot decompose ![]() to
give
to
give ![]() gas.
gas.
![]() No
reaction
(d) When
quick lime (CaO) is added to water in a test tube. Some of it will dissolve to
form calcium hydroxide (lime water) while majority of it remains suspended.
Filter the solution. The clear solution thus obtained is called lime water.
No
reaction
(d) When
quick lime (CaO) is added to water in a test tube. Some of it will dissolve to
form calcium hydroxide (lime water) while majority of it remains suspended.
Filter the solution. The clear solution thus obtained is called lime water.
![]()
You need to login to perform this action.
You will be redirected in
3 sec
