Answer:
(i) Since, compound ![]() contains
two oxygen atoms, therefore most probably it may be a carboxylic acid, i.e,
ethanoic acid
contains
two oxygen atoms, therefore most probably it may be a carboxylic acid, i.e,
ethanoic acid ![]() .
(ii) Since, an acid,
i.e. ,ethanoic acid reacts with a base, i.e., Na metal to evolve a gas which
burns with a pop sound along with the formation of compound (R), therefore, fl
must be a salt, i'.e., sodium ethanoate and the gas which burns with a pop sound
must be
.
(ii) Since, an acid,
i.e. ,ethanoic acid reacts with a base, i.e., Na metal to evolve a gas which
burns with a pop sound along with the formation of compound (R), therefore, fl
must be a salt, i'.e., sodium ethanoate and the gas which burns with a pop sound
must be ![]() gas.
gas.


![]() gas
burns with 'pop' sound.
Compound R
is sodium ethanoate. This is also supported by the observation that when ethanoic
acid reacts with NaOH, it gives R, is sodium ethanoate and water.
gas
burns with 'pop' sound.
Compound R
is sodium ethanoate. This is also supported by the observation that when ethanoic
acid reacts with NaOH, it gives R, is sodium ethanoate and water.
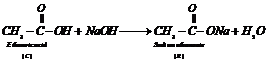
![]()
![]() Since
compound C on treatment with an alcohol A in presence of acid forms a sweet smelling
compound C(M.F.
Since
compound C on treatment with an alcohol A in presence of acid forms a sweet smelling
compound C(M.F.![]() ),
therefore, S is methyl ethanoate (ester).Since, ester S has three carbon atoms
and the acid C has two carbon atoms, therefore alcohol A must contain one C
atom, i.e., A is methanol.
),
therefore, S is methyl ethanoate (ester).Since, ester S has three carbon atoms
and the acid C has two carbon atoms, therefore alcohol A must contain one C
atom, i.e., A is methanol.
![]() Thus,
Thus,
![]()
![]()
![]()
![]()
You need to login to perform this action.
You will be redirected in
3 sec
