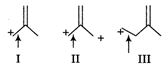
A) I > II > III
B) II > I > III
C) III > II > I
D) I > III > II
Correct Answer: C
Solution :
The length of carbon-carbon single bond is always greater than that of the carbon-carbon double bond. In III, positive charge is not in conjugation of double bond, so the bond does not acquire a double bond character. However, chances of acquiring double bond character (of the indicated bond) is much more in I than that in case of II. Thus, the correct order of decreasing bond length is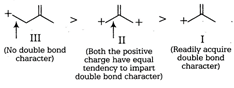
You need to login to perform this action.
You will be redirected in
3 sec
