A) \[C{{H}_{3}}CHO\]
B) \[PhCOC{{H}_{3}}\]
C) \[PhCOPh\]
D) \[C{{H}_{3}}COC{{H}_{3}}\]
Correct Answer: A
Solution :
Carbonyl compounds undergoes nucleophilic addition reaction.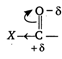 [Y- shows negative inductive effect] If group or atom attached with carbonyl carbon shows negative inductive effect, then it decreases electron density on carbonyl carbon and facilitate the attack of nucleophiles, hence reactivity of carbonyl compound increases.
[Y- shows negative inductive effect] If group or atom attached with carbonyl carbon shows negative inductive effect, then it decreases electron density on carbonyl carbon and facilitate the attack of nucleophiles, hence reactivity of carbonyl compound increases. You need to login to perform this action.
You will be redirected in
3 sec
