A) 1
B) 4
C) 2
D) 6
Correct Answer: D
Solution :
\[E={{E}_{1}}/{{n}^{2}}\] Energy used for excitation is 12.75 eV ie, \[(-13.6+12.75)eV=-0.85\,eV\]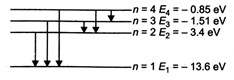 Energy levels of H-atom The photons of energy 12.75 eV can excite the fourth level of H-atom. Therefore, six lines will be emitted, \[\left( n\left( \frac{(n-1)}{2} \right)\text{lines} \right).\]
Energy levels of H-atom The photons of energy 12.75 eV can excite the fourth level of H-atom. Therefore, six lines will be emitted, \[\left( n\left( \frac{(n-1)}{2} \right)\text{lines} \right).\]
You need to login to perform this action.
You will be redirected in
3 sec
