A) \[+\,110\,k\,mo{{l}^{-1}}\]
B) \[-\,110\,kJ\,mo{{l}^{-1}}\]
C) \[+\,610\,kJ\,mo{{l}^{-1}}\]
D) \[-\,610\,kJ\,mo{{l}^{-1}}\]
E) None option is correct
Correct Answer: E
Solution :
\[\Delta H={{E}_{f}}-{{E}_{b}}=250-360\,kJ=110\,kJ.\]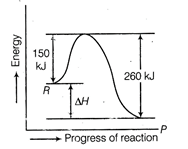
You need to login to perform this action.
You will be redirected in
3 sec
